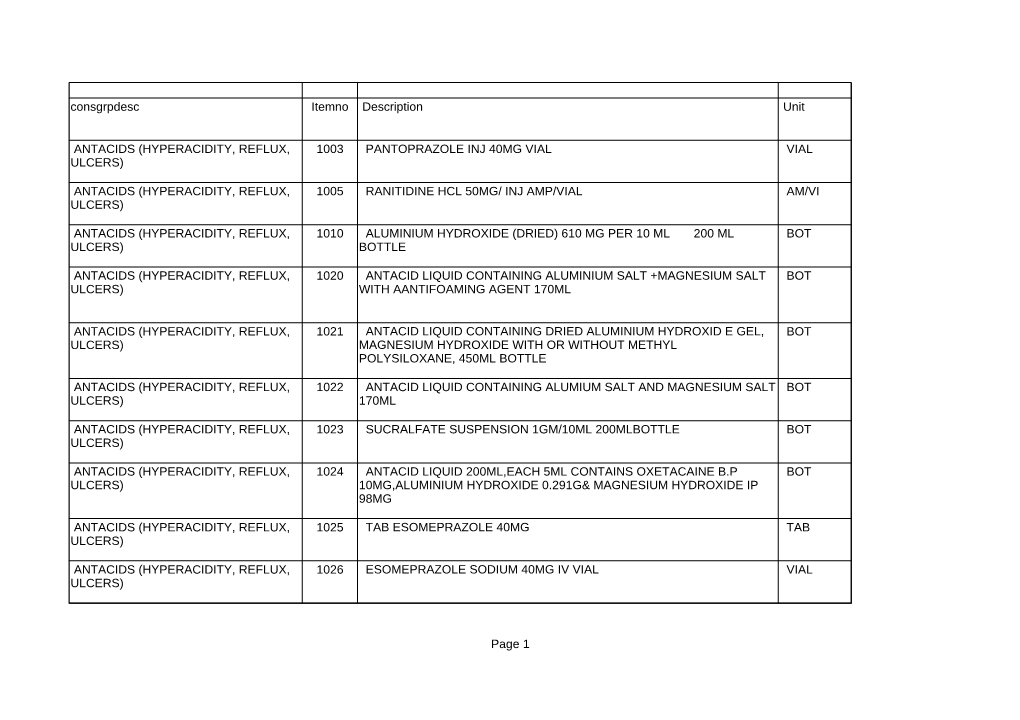
Page 1 Consgrpdesc Itemno Unit 1003 VIAL 1005 AM/VI 1010 BOT 1020 BOT 1021 BOT 1022 BOT 1023 BOT 1024 BOT
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

30 Day Change Notice Effective Date
30 Day Change Notice Effective Date: January 1st, 2021 NEW PREFERRED DRUGS THERAPEUTIC CLASS NO PA REQUIRED PREFERRED Central Nervous System (CNS) Agents: Anticonvulsants Clobazam (Generic of Onfi) Central Nervous System (CNS) Agents: Multiple Aubagio EndocrineSclerosis Agents: Osteoporosis-Bone Ossification Forteo Enhancers Gastrointestinal Agents: Anti-Emetics Bonjesta Genitourinary Agents: Benign Prostatic Hyperplasia Alfuzosin (Generic of Uroxatral) Dutasteride (Generic of Avodart) Genitourinary Agents: Electrolyte Depleter Agents Sevelamer (Generic of Renagel and Renvela) Infectious Disease Agents: Antibiotics-Macrolides Eryped Infectious Disease Agents: Antivirals-HIV Atazanavir Sulfate Oral Powder (Generic of Reyataz) Tivicay PD Infectious Disease Agents: Antibiotics-Tetracyclines Vibramycin Suspension (no PA Required for age 12 or under) Ophthalmic Agents: Antibiotics and Antibiotic -Steroid Neomycin/Polymyxin/Bacitracin/Hydrocortisone Ointment Combination Drops and Ointments Ophthalmic Agents: Glaucoma Agents Dorzolamide/Timolol (Generic of Cosopt PF) NEW CLINICAL PA REQUIRED “PREFERRED” DRUGS THERAPEUTIC CLASS CLINICAL PA REQUIRED PREFERRED Blood Formation, Coagulation, and Thrombosis Agents: Corifact Hemophilia Factors Immunomodulator Agents for Systemic Inflammatory Taltz Disease Immunomodulator Agents for Systemic Inflammatory Xeljanz 5mg Disease NEW STEP THERAPY REQUIRED “PREFERRED” THERAPEUTIC CLASS STEP THERAPY REQUIRED “PREFERRED” Central nervous System (CNS) Agents: Anti-Migraine, Aimovig Prophylaxis Treatment Ajovy -

Active Pharmaceutical Ingredients
Active Pharmaceutical Ingredients Catalog HPD-5E ® CREATING A HEALTHY WORLDTM Active Pharmaceutical Ingredients (APIs) Available for International Markets Human Pharmaceutical Department www.Pharmapex.net Catalog HPD-5E *Not all products referred to on this site are available in all countries and our products are subject to different regulatory requirements depending on the country of use. Consequently, certain sections of this site may be indicated as being intended only for users in specic countries. Some of the products may also be marketed under different trade names. You should not construe anything on this site as a promotion or solicitation for any product or for the use of any product that is not authorized by the laws and regulations of your country of residence. For inquiries about the availability of any specic product in your country, you may simply contact us at [email protected]. **Products currently covered by valid US Patents may be offered for R&D use in accordance with 35 USC 271(e)+A13(1). Any patent infringement and resulting liability is solely at buyer risk. ©2016, Pharmapex USA, A member of Apex Group of Companies, All Rights Reserved. Toll-Free: 1.844.PHARMAPEX Fax: + 1.619.881.0035 ACTIVE PHARMACEUTICAL [email protected] CREATING A HEALTHY WORLD™ www.Pharmapex.net INGREDIENTS About Pharmapex’s Human Pharmaceuticals Department: Pharmapex’s Human Pharmaceuticals Department (HPD) is a leading source for high-quality Active Pharmaceutical Ingredients (APIs) and Finished Pharmaceutical Products (FPPs) in various markets across the globe. With an extensive product portfolio, our consortium of companies is dedicated to addressing and solving the most important medical needs of our time, including oncology (e.g., multiple myeloma and prostate cancer), neuroscience (e.g., schizophrenia, dementia and pain), infectious disease (e.g., HIV/AIDS, Hepatitis C and tuberculosis), and cardiovascular and metabolic diseases (e.g., diabetes). -

Tanibirumab (CUI C3490677) Add to Cart
5/17/2018 NCI Metathesaurus Contains Exact Match Begins With Name Code Property Relationship Source ALL Advanced Search NCIm Version: 201706 Version 2.8 (using LexEVS 6.5) Home | NCIt Hierarchy | Sources | Help Suggest changes to this concept Tanibirumab (CUI C3490677) Add to Cart Table of Contents Terms & Properties Synonym Details Relationships By Source Terms & Properties Concept Unique Identifier (CUI): C3490677 NCI Thesaurus Code: C102877 (see NCI Thesaurus info) Semantic Type: Immunologic Factor Semantic Type: Amino Acid, Peptide, or Protein Semantic Type: Pharmacologic Substance NCIt Definition: A fully human monoclonal antibody targeting the vascular endothelial growth factor receptor 2 (VEGFR2), with potential antiangiogenic activity. Upon administration, tanibirumab specifically binds to VEGFR2, thereby preventing the binding of its ligand VEGF. This may result in the inhibition of tumor angiogenesis and a decrease in tumor nutrient supply. VEGFR2 is a pro-angiogenic growth factor receptor tyrosine kinase expressed by endothelial cells, while VEGF is overexpressed in many tumors and is correlated to tumor progression. PDQ Definition: A fully human monoclonal antibody targeting the vascular endothelial growth factor receptor 2 (VEGFR2), with potential antiangiogenic activity. Upon administration, tanibirumab specifically binds to VEGFR2, thereby preventing the binding of its ligand VEGF. This may result in the inhibition of tumor angiogenesis and a decrease in tumor nutrient supply. VEGFR2 is a pro-angiogenic growth factor receptor -

Anatomy and Physiology of Peritoneal Dialysis
Anatomy and Physiology of Peritoneal Dialysis Isaac Teitelbaum, MD Professor of Medicine Director, Acute & Home Dialysis Programs University of Colorado Hospital Denver, Colorado •1 Outline • Peritoneal cavity as a dialysis system • Models of peritoneal transport • Physiology of peritoneal transport Inverse relationship between solute transport and ultrafiltration • Kinetics of peritoneal transport • Synthesis & Application • Middle Molecules Anatomy of The Peritoneum • The lining of the abdominal cavity • Two layers: parietal - lines the anterior wall and undersurface of the diaphragm - 20% of total SA; blood supply from abdominal wall visceral - covers the abdominal organs - 80% of total SA; blood supply from mesenteric aa and portal vv Gokal R, Textbook of PD, pp. 61-70 •3 Anatomy of The Peritoneum • Size 1.5 – 2 m2; approximates BSA • Highly Vascular • Semi-permeable/bi-directional • “Lymphatic” drainage through diaphragmatic stomata • Continuous with Fallopian Tubes in females Gokal R, Textbook of PD, pp. 61-70 1. The two main properties of the peritoneal membrane are: a. Semi permeable – this allows substances of certain sizes to move from an area of greater concentration to less concentration. b. Bi Directional - substances move in either direction across the membrane. 2. So-called “lymphatic” drainage refers to bulk flow from the peritoneal cavity back to the circulation. This actually occurs across tissues as well as lymphatics. As this is convective flow, dissolved solutes move with the fluid. Thus, fluid reabsorption results in loss of solute clearance as well as loss of fluid removal. 3. It is important to be aware of the continuity of the peritoneal cavity with the Fallopian tubes as retrograde menstruation- which may occur in any woman but goes undetected- will cause bloody dialysate and create concern in the PD patient. -

INTEGRIS Formulary July 2017
INTEGRIS Formulary July 2017 Foreword FORMULARY EXCLUDED THERAPEUTIC DRUG This document represents the efforts of the MedImpact Healthcare Systems THERAPEUTIC DRUGS CLASS Pharmacy and Therapeutics (P & T) and Formulary Committees to provide ALTERNATIVES physicians and pharmacists with a method to evaluate the safety, efficacy and cost- clindamycin/tretinoin, ACNE AGENTS, effectiveness of commercially available drug products. A structured approach to the VELTIN drug selection process is essential in ensuring continuing patient access to rational ZIANA TOPICAL drug therapies. The ultimate goal of the MedPerform Formulary is to provide a morphine sulfate ER process and framework to support the dynamic evolution of this document to guide tablets, oxycodone ANALGESICS, KADIAN prescribing decisions that reflect the most current clinical consensus associated ER, NUCYNTA, NARCOTICS with drug therapy decisions. NUCYNTA ER ANALGESICS, This is accomplished through the auspices of the MedImpact P & T and Formulary BELBUCA BUTRANS PATCH Committees. These committees meet quarterly and more often as warranted to NARCOTICS ensure clinical relevancy of the Formulary. To accommodate changes to this ABSTRAL, document, updates are made accessible as necessary. FENTORA, fentanyl citrate ANALGESICS, LAZANDA, lozenge NARCOTICS As you use this Formulary, you are encouraged to review the information and ONSOLIS, provide your input and comments to the MedImpact P & T and Formulary SUBSYS Committees. immediate-release GRALISE ANTICONVULSANTS The MedImpact P & T -

Comparison of Clinical Effects Between
Kanno et al. Renal Replacement Therapy (2020) 6:7 https://doi.org/10.1186/s41100-019-0253-4 REVIEW Open Access Comparison of clinical effects between icodextrin and glucose solutions on outcomes of peritoneal dialysis: systematic review and meta-analysis of randomized controlled trials Atsuhiro Kanno1*, Yasushi Tsujimoto2, Takayuki Fujii3, Emi Fujikura4, Kimio Watanabe5, Hidemichi Yuasa6, Munekazu Ryuzaki7, Yasuhiko Ito8 and Hidetomo Nakamoto9 Abstract Background: Icodextrin enhances peritoneal filtration for patients on peritoneal dialysis (PD). However, clinically important outcomes have not yet been analyzed using authentic, objective statistical methods. The present systematic review aimed to determine the risks and benefits of icodextrin compared with a glucose-based solution with respect to clinically important and patient-centered outcomes. Methods: We systematically investigated only randomized controlled trials (RCTs) by adopting the Cochrane Database of Systematic Review (2014) and searched the CENTRAL, MEDLINE, and EMBASE databases for eligible studies reported in the literature. The quality of the evidence was assessed using the GRADE approach. Results: We finally evaluated important outcomes in 13 RCTs. Icodextrin significantly decreased the number of reported episodes of uncontrolled fluid overload in four RCTs that involved 236 patients (relative risk [RR], 0.31; 95% confidence interval [CI], 0.12 to 0.82; moderate certainty evidence). However, the inclusion of icodextrin for peritoneal ultrafiltration did not significantly differ in six RCTs involving 252 patients (mean difference [MD], 186.76 mL; 95% CI, − 47.08 to 420.59; low certainty evidence). Regarding other clinically important outcomes, all-cause mortality in 10 RCTs involving 1106 patients (RR, 0.75; 95% CI, 0.33 to 1.71; low certainty evidence) and technical survival in five RCTs involving 470 patients (RR, 0.57; 95%CI, 0.29 to 1.12; low certainty evidence) were not significant. -

Printed Formulary Catalog Basic
Scripps Health Formulary July 2016 Foreword Pharmacy and Therapeutics Committee. MedImpact approves such multi- This document represents the efforts of the MedImpact Healthcare Systems source drugs for addition to the MAC list based on the following criteria: Pharmacy and Therapeutics (P & T) and Formulary Committees to provide physicians A multi-source drug product manufactured by at least one (1) nationally and pharmacists with a method to evaluate the safety, efficacy and cost-effectiveness marketed company. of commercially available drug products. A structured approach to the drug selection At least one (1) of the generic manufacturer’s products must have an “A” process is essential in ensuring continuing patient access to rational drug therapies. rating or the generic product has been determined to be unassociated with The ultimate goal of the Portfolio Formulary is to provide a process and framework to efficacy, safety or bioequivalency concerns by the MedImpact P & T support the dynamic evolution of this document to guide prescribing decisions that Committee. reflect the most current clinical consensus associated with drug therapy decisions. Drug product will be approved for generic substitution by the MedImpact P & T Committee. This is accomplished through the auspices of the MedImpact P & T and Formulary Committees. These committees meet quarterly and more often as warranted to ensure This list is reviewed and updated periodically based on the clinical literature and clinical relevancy of the Formulary. To accommodate changes to this document, pharmacokinetic characteristics of currently available versions of these drug updates are made accessible as necessary. products. As you use this Formulary, you are encouraged to review the information and provide If a member or physician requests a brand name product in lieu of an approved your input and comments to the MedImpact P & T and Formulary Committees. -

Malta Medicines List April 08
Defined Daily Doses Pharmacological Dispensing Active Ingredients Trade Name Dosage strength Dosage form ATC Code Comments (WHO) Classification Class Glucobay 50 50mg Alpha Glucosidase Inhibitor - Blood Acarbose Tablet 300mg A10BF01 PoM Glucose Lowering Glucobay 100 100mg Medicine Rantudil® Forte 60mg Capsule hard Anti-inflammatory and Acemetacine 0.12g anti rheumatic, non M01AB11 PoM steroidal Rantudil® Retard 90mg Slow release capsule Carbonic Anhydrase Inhibitor - Acetazolamide Diamox 250mg Tablet 750mg S01EC01 PoM Antiglaucoma Preparation Parasympatho- Powder and solvent for solution for mimetic - Acetylcholine Chloride Miovisin® 10mg/ml Refer to PIL S01EB09 PoM eye irrigation Antiglaucoma Preparation Acetylcysteine 200mg/ml Concentrate for solution for Acetylcysteine 200mg/ml Refer to PIL Antidote PoM Injection injection V03AB23 Zovirax™ Suspension 200mg/5ml Oral suspension Aciclovir Medovir 200 200mg Tablet Virucid 200 Zovirax® 200mg Dispersible film-coated tablets 4g Antiviral J05AB01 PoM Zovirax® 800mg Aciclovir Medovir 800 800mg Tablet Aciclovir Virucid 800 Virucid 400 400mg Tablet Aciclovir Merck 250mg Powder for solution for inj Immunovir® Zovirax® Cream PoM PoM Numark Cold Sore Cream 5% w/w (5g/100g)Cream Refer to PIL Antiviral D06BB03 Vitasorb Cold Sore OTC Cream Medovir PoM Neotigason® 10mg Acitretin Capsule 35mg Retinoid - Antipsoriatic D05BB02 PoM Neotigason® 25mg Acrivastine Benadryl® Allergy Relief 8mg Capsule 24mg Antihistamine R06AX18 OTC Carbomix 81.3%w/w Granules for oral suspension Antidiarrhoeal and Activated Charcoal -

Index B Alphabetical List of Pharmaceutical Product Names
INDEX B ALPHABETICAL LIST OF PHARMACEUTICAL PRODUCT NAMES PRODUCT NAME Page PRODUCT NAME Page 292 97 ACYCLOVIR 17 3TC (EDS) 14 ACYCLOVIR 17 5-AMINOSALICYLIC ACID (MESALAMINE) 170 " 18 ABACAVIR SO4 13 ADALAT XL 57 ABACAVIR SO4/LAMIVUDINE 13 ADALIMUMAB 220 ABACAVIR SO4/LAMIVUDINE/ZIDOVUDINE 13 ADAPALENE 206 ABATACEPT 220 ADCIRCA (EDS) 89 ABILIFY (EDS) 123 ADEFOVIR DIPIVOXIL 18 ACARBOSE 187 ADHESIVE WIPES (EDS) 239 ACCEL PIOGLITAZONE (EDS) 190 ADVAGRAF (EDS) 231 ACCEL TOPIRAMATE 112 ADVAIR (EDS) 33 ACCEL-CITALOPRAM 114 ADVAIR DISKUS (EDS) 33 ACCOLATE (EDS) 232 AGGRENOX (EDS) 87 ACCU-CHEK 1 MINI (EDS) 238 AGRYLIN 221 ACCU-CHEK 2 MINI (EDS) 238 AIROMIR 32 ACCU-CHEK ADVANTAGE 142 ALCOHOL PREP 234 ACCU-CHEK AVIVA 142 ALCOHOL SWAB 234 ACCU-CHEK COMPACT 142 ALDACTAZIDE-25 82 ACCU-CHEK FASTCLIX MOBILE 234 ALDACTAZIDE-50 82 ACCU-CHEK LINKASSIST (EDS) 239 ALDACTONE 148 ACCU-CHEK MOBILE 142 ALDARA (EDS) 209 ACCU-CHEK MULTICLIX 234 ALENDRONATE (EDS) 220 ACCU-CHEK RAPID D (EDS) 236 ALENDRONATE SODIUM 220 " 237 ALENDRONATE SODIUM (EDS) 220 " 238 ALENDRONATE SODIUM/VITAMIN D3 ACCU-CHEK SPIRIT (EDS) 236 (CHOLECALCIFEROL) 220 ACCU-CHEK TENDER 1 (EDS) 238 ALERTEC (EDS) 133 " 239 ALESSE 181 ACCU-CHEK TENDER 2 (EDS) 238 ALFACALCIDOL 217 " 239 ALFUZOSIN 220 ACCU-CHEK ULTRAFLEX 1 (EDS) 237 ALGLUCOSIDASE ALFA 221 " 238 ALLOPURINOL 221 ACCU-CHEK ULTRAFLEX 2 (EDS) 237 ALMOTRIPTAN MALATE 33 " 238 ALOMIDE 158 ACCUPRIL 79 ALPHAGAN 157 ACCURETIC 80 ALPHAGAN P 157 ACCUTANE 209 ALPRAZOLAM 134 ACCUTREND 142 ALPRAZOLAM 134 ACEBUTOLOL 48 ALTACE 80 ACEBUTOLOL HCL 48 " 81 -

Pharmacokinetics of Ophthalmic Corticosteroids
British Journal ofOphthalmology 1992; 76: 681-684 681 MINI REVIEW Br J Ophthalmol: first published as 10.1136/bjo.76.11.681 on 1 November 1992. Downloaded from Pharmacokinetics of ophthalmic corticosteroids Corticosteroids have been used by ophthalmologists with an identical vehicle, the aqueous humour concentrations of increasing frequency over the past 30 years, with the these steroids are almost identical.'9 None the less it is concomitant development of a diverse range of drop, essential when considering such empirical data, to recall that ointment, subconjunctival, and oral preparations. Though the systemic anti-inflammatory effect of both betamethasone the clinical benefits and side effects of such corticosteroid and dexamethasone is five to seven times that of predniso- preparations have been well documented, their basic lone.39"' The local anti-inflammatory potency of ocular pharmacokinetics in the human eye have yet to be fully steroids has yet to be fully investigated and whilst early work established. Indeed most of our pharmacokinetic knowledge suggested that prednisolone acetate 1% had the greatest anti- of these drugs has been elucidated by extrapolation of data inflammatory effect in experimental keratitis,'7 later studies obtained from rabbit experiments.1-26 These results can be demonstrated that fluorometholone acetate in a 1% formu- significantly disparate from human data because of the lation was equally efficacious in the same model.26 However, thinner rabbit cornea, lower rabbit blink rate, effect of prednisolone -

Fluid and Pharmacological Agents for Adhesion Prevention After Gynaecological Surgery (Review)
Fluid and pharmacological agents for adhesion prevention after gynaecological surgery (Review) Ahmad G, Mackie FL, Iles DA, O’Flynn H, Dias S, Metwally M, Watson A This is a reprint of a Cochrane review, prepared and maintained by The Cochrane Collaboration and published in The Cochrane Library 2014, Issue 7 http://www.thecochranelibrary.com Fluid and pharmacological agents for adhesion prevention after gynaecological surgery (Review) Copyright © 2014 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd. TABLE OF CONTENTS HEADER....................................... 1 ABSTRACT ...................................... 1 PLAINLANGUAGESUMMARY . 2 SUMMARY OF FINDINGS FOR THE MAIN COMPARISON . ..... 4 BACKGROUND .................................... 6 OBJECTIVES ..................................... 7 METHODS ...................................... 7 RESULTS....................................... 9 Figure1. ..................................... 10 Figure2. ..................................... 12 Figure3. ..................................... 13 Figure4. ..................................... 14 Figure5. ..................................... 15 Figure6. ..................................... 16 Figure7. ..................................... 17 Figure8. ..................................... 18 Figure9. ..................................... 18 Figure10. ..................................... 19 ADDITIONALSUMMARYOFFINDINGS . 22 DISCUSSION ..................................... 32 AUTHORS’CONCLUSIONS . 33 ACKNOWLEDGEMENTS . 33 REFERENCES .................................... -

Teva Pharmaceutical Industries Ltd
m n a d v s g o PRODUCT v1 2015 CATALOGUE h p b e q f i t w c l r z www.tapi.com PRODUCT CATALOGUE v1 2015 US CEP EU Japan Korea DMF DMF DMF DMF ABIRATERONE a ACYCLOVIR x x x AFATINIB ALCLOMETASONE DIPROPIONATE x x ALENDRONATE SODIUM x x x ALLOPURINOL x x x x AMCINONIDE x x x AMITRIPTYLINE HCL x x x x x AMLODIPINE BESYLATE x x x ANASTROZOLE x x x ANIDULAFUNGIN APIXABAN APREMILAST ARIPIPRAZOLE x x ARIPIPRAZOLE LAUROXIL ATORVASTATIN CALCIUM* x x x x ATOSIBAN ACETATE ATRACURIUM BESYLATE x x x AZACITIDINE x x AZITHROMYCIN x x x x AZTREONAM x BECLOMETHASONE DIPROPIONATE x x x x b BETAMETHASONE ACETATE x x x BETAMETHASONE BASE x x x BETAMETHASONE DIPROPIONATE x x x x BETAMETHASONE VALERATE x x x x BICALUTAMIDE x x BIMATOPROST x x BIVALIRUDIN x x BLEOMYCIN SULFATE x x BORTEZOMIB x x x * TAPI can provide several different polymorphs or processes of this product. To obtain complete DMF files of a specific product's polymorph or process, please contact your account manager. For the most updated product list please go to "Products" at www.tapi.com Additional DMFs or Tech Files are available upon request All subject to the disclaimer on page 15 PRODUCT CATALOGUE v1 2015 US CEP EU Japan Korea DMF DMF DMF DMF BROMOCRIPTINE MESYLATE x x b BUDESONIDE x x x x BUPRENORPHINE BASE x x BUPRENORPHINE HCL x x x BUTORPHANOL TARTRATE x x CABAZITAXEL x CABERGOLINE x x x x c CALCIPOTRIENE (CALCIPOTRIOL)* x x x CALCITRIOL x x x CANDESARTAN CILEXETIL x CAPECITABINE x x CARBIDOPA x x x x CARBOPLATIN x x x x CARFILZOMIB CARVEDILOL BASE x x x x x CARVEDILOL PHOSPHATE x CASPOFUNGIN ACETATE x x CERITINIB CHLORMADINONE ACETATE x x CICLOSPORIN x x x x x CILOSTAZOL x x x x CINACALCET HCL x x CISATRACURIUM BESYLATE x x CISPLATIN x x x CLARITHROMYCIN x x x x x CLOBETASOL PROPIONATE x x x x x CLONAZEPAM x CLOPIDOGREL BISULFATE x x x x CYPROTERONE ACETATE x x d DABIGATRAN ETEXILATE x DARIFENACIN HBR x x * TAPI can provide several different polymorphs or processes of this product.