Basking and Torpor in a Rock-Dwelling Desert Marsupial: Survival Strategies in a Resource-Poor Environment
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Platypus Collins, L.R
AUSTRALIAN MAMMALS BIOLOGY AND CAPTIVE MANAGEMENT Stephen Jackson © CSIRO 2003 All rights reserved. Except under the conditions described in the Australian Copyright Act 1968 and subsequent amendments, no part of this publication may be reproduced, stored in a retrieval system or transmitted in any form or by any means, electronic, mechanical, photocopying, recording, duplicating or otherwise, without the prior permission of the copyright owner. Contact CSIRO PUBLISHING for all permission requests. National Library of Australia Cataloguing-in-Publication entry Jackson, Stephen M. Australian mammals: Biology and captive management Bibliography. ISBN 0 643 06635 7. 1. Mammals – Australia. 2. Captive mammals. I. Title. 599.0994 Available from CSIRO PUBLISHING 150 Oxford Street (PO Box 1139) Collingwood VIC 3066 Australia Telephone: +61 3 9662 7666 Local call: 1300 788 000 (Australia only) Fax: +61 3 9662 7555 Email: [email protected] Web site: www.publish.csiro.au Cover photos courtesy Stephen Jackson, Esther Beaton and Nick Alexander Set in Minion and Optima Cover and text design by James Kelly Typeset by Desktop Concepts Pty Ltd Printed in Australia by Ligare REFERENCES reserved. Chapter 1 – Platypus Collins, L.R. (1973) Monotremes and Marsupials: A Reference for Zoological Institutions. Smithsonian Institution Press, rights Austin, M.A. (1997) A Practical Guide to the Successful Washington. All Handrearing of Tasmanian Marsupials. Regal Publications, Collins, G.H., Whittington, R.J. & Canfield, P.J. (1986) Melbourne. Theileria ornithorhynchi Mackerras, 1959 in the platypus, 2003. Beaven, M. (1997) Hand rearing of a juvenile platypus. Ornithorhynchus anatinus (Shaw). Journal of Wildlife Proceedings of the ASZK/ARAZPA Conference. 16–20 March. -

Impact of Fox Baiting on Tiger Quoll Populations Project ID: 00016505
Impact of fox baiting on tiger quoll populations Project ID: 00016505 Final Report to Environment Australia and The New South Wales National Parks and Wildlife Service Gerhard Körtner and Shaan Gresser Copyright G. Körtner Executive Summary: The NSW Threat Abatement Plan for Predation by the Red Fox (TAP) identifies foxes as a major threat to the survival of many native mammals. The plan recommends baiting with compound 1080 (sodium monofluoroacetate) because it appears to be the most effective fox control measure. However, the plan also recognises the risk for tiger quolls as a non-target species. Although the actual impact of 1080 fox baiting on tiger quoll populations has not been assessed, this assumed risk has resulted in restrictions on the use of 1080 which render fox baiting programs labour intensive and expensive and which may compromise the effectiveness of the fox control. The aim of this project is to determine whether these precautions are necessary by measuring tiger quoll mortality during fox baiting programs using 1080. The project has been identified as a priority action (Obj. 2, action 5) of the TAP. Three experiments were conducted in north-east NSW between June 2000 and December 2001. Overall 78 quolls were trapped and 56 of those were fitted with mortality radio-transmitters. Baiting procedure followed Best Practice Guidelines (TAP) except that there was no free-feeding and baits were only surface buried. These modifications aimed to increase the exposure of quolls to bait. 1080 baits (3 mg / bait; Foxoff®) incorporating the bait marker Rhodamine B were deployed for 10 days along existing trails. -

Ba3444 MAMMAL BOOKLET FINAL.Indd
Intot Obliv i The disappearing native mammals of northern Australia Compiled by James Fitzsimons Sarah Legge Barry Traill John Woinarski Into Oblivion? The disappearing native mammals of northern Australia 1 SUMMARY Since European settlement, the deepest loss of Australian biodiversity has been the spate of extinctions of endemic mammals. Historically, these losses occurred mostly in inland and in temperate parts of the country, and largely between 1890 and 1950. A new wave of extinctions is now threatening Australian mammals, this time in northern Australia. Many mammal species are in sharp decline across the north, even in extensive natural areas managed primarily for conservation. The main evidence of this decline comes consistently from two contrasting sources: robust scientifi c monitoring programs and more broad-scale Indigenous knowledge. The main drivers of the mammal decline in northern Australia include inappropriate fi re regimes (too much fi re) and predation by feral cats. Cane Toads are also implicated, particularly to the recent catastrophic decline of the Northern Quoll. Furthermore, some impacts are due to vegetation changes associated with the pastoral industry. Disease could also be a factor, but to date there is little evidence for or against it. Based on current trends, many native mammals will become extinct in northern Australia in the next 10-20 years, and even the largest and most iconic national parks in northern Australia will lose native mammal species. This problem needs to be solved. The fi rst step towards a solution is to recognise the problem, and this publication seeks to alert the Australian community and decision makers to this urgent issue. -

Spotted Tailed Quoll (Dasyurus Maculatus)
Husbandry Guidelines for the SPOTTED-TAILED QUOLL (Tiger Quoll) (Photo: J. Marten) Dasyurus maculatus (MAMMALIA: DASYURIDAE) Author: Julie Marten Date of Preparation: February 2013 – June 2014 Western Sydney Institute of TAFE, Richmond Course Name and Number: Captive Animals Certificate III (18913) Lecturers: Graeme Phipps, Jacki Salkeld, Brad Walker DISCLAIMER Please note that this information is just a guide. It is not a definitive set of rules on how the care of Spotted- Tailed Quolls must be conducted. Information provided may vary for: • Individual Spotted-Tailed Quolls • Spotted-Tailed Quolls from different regions of Australia • Spotted-Tailed Quolls kept in zoos versus Spotted-Tailed Quolls from the wild • Spotted-Tailed Quolls kept in different zoos Additionally different zoos have their own set of rules and guidelines on how to provide husbandry for their Spotted-Tailed Quolls. Even though I researched from many sources and consulted various people, there are zoos and individual keepers, researchers etc. that have more knowledge than myself and additional research should always be conducted before partaking any new activity. Legislations are regularly changing and therefore it is recommended to research policies set out by national and state government and associations such as ARAZPA, ZAA etc. Any incident resulting from the misuse of this document will not be recognised as the responsibility of the author. Please use at the participants discretion. Any enhancements to this document to increase animal care standards and husbandry techniques are appreciated. Otherwise I hope this manual provides some helpful information. Julie Marten Picture J.Marten 2 OCCUPATIONAL HEALTH AND SAFETY RISKS It is important before conducting any work that all hazards are identified. -

Adec Preview Generated PDF File
Rec. West. Aust. Mus. 1988. 14(1): 61-71 A new species of false antechinus (Marsupialia: Dasyuridae) from the Kimberley, Western Australia D.J. Kitchener* Abstract Pseudantechinus ningbing sp. nov., recognised as a species in literature, is herein formally described. It is widely distributed in the Kimberley district, Western Aust ralia. Introduction The false antechinusus or Parantechini sensu Archer, 1982 were recognised as a distinct group on the basis of isozyme electrophoresis by Baverstock et al. (1982), who included within this group the following Antechinus species: A. macdonnellen sis (Spencer, 1896); A. bilarni Johnson, 1964 and A. rosamondae Ride, 1964. Baverstock et al. (1982) suggested that the form referred to as 'ningbing' probably belonged to this group. Archer (1982), using essentially cranial and several external characters, considered that the Parantechini comprised three genera. He recognised, but did not formally describe, the 'ningbing' form as a species and placed it in Pseudantechinus Tate, 1947 - which he considered to also include macdonnellensis. Woolley (1982), on the basis of phallic morphology, also recognised the 'ningbing' form and placed it in the Parantechini. Cooper and Woolley (1983) examined the electrophoretic mobility of proteins and enzymes of eight species of dasyurid marsupials, including the 'ningbing' form, and concluded that 'ningbing' was probably a species. Kitchener and Caputi (1988) described the species Pseudantechinus woolleyae and also recognised Pseudantechinus 'ningbing' as a species. They concluded that P. 'ningbing' was sexually dimorphic, it was phenetically closest to Pseudan techinus macdonnellensis and Pseudantechinus bilarni, and phylogenetically closest to P. macdonnellensis, P. bilarni and P. woolleyae, but could not resolve the relationships between these Pseudantechinus species. -

For Saving Species Winter 2018 Issue 8 Saving the Brush-Tailed Rabbit-Rat
Science for saving species Winter 2018 Issue 8 Saving the brush-tailed rabbit-rat Australia’s most imperilled animals Protecting an elusive dunnart Bioaccoustic monitoring Booderee’s mammal puzzle Bundles of quoll joy IMAGE: BY JOHN DAVIES Magazine of the Threatened Species Recovery Hub No surprises, no regrets: identifying Australia’s most imperilled animal species It was only in 1929 that thylacines were first afforded any protection under legislation. Seven years later it was added to the list of protected wildlife, but the last known individual died that same year. The Christmas Island forest skink was first included on Australia’s list of threatened species in January 2014. Just four months later, the last known individual died. Both extinctions could almost certainly have been prevented if action had been taken earlier. The gnawing question ‘what if we had known earlier...?’ is a recurring theme of frustration and failure in much conservation biology – as it is in human experience generally. When recognition of the imminence of a serious and irretrievable loss is belated, opportunities for better outcomes are fatally lost. The trajectory and timetable of species to species, and their degree of confidence in extinction was looming larger for the extinction is at least partly predictable. that assessment. Estimates were pooled most imperilled birds than for mammals. To provide forewarnings, a TSR Hub project, across experts, with weighting by this This may be because many of the most is identifying the Australian animal species confidence level. From this information, imperilled mammals have had some recent at greatest risk and estimating the likelihoods we ordered species by their likelihood of reprieves through translocations to that they will become extinct over the next extinction, and then summed these estimates predator exclosures and cat-free islands. -

Wildlife Matters Wildlife Conservancy
australian wildlife matters wildlife conservancy Spring 2009 Pungalina reveals one of Australia’s rarest mammals Carpentarian Pseudantechinus 2 australian saving australia’s threatened wildlife wildlife Pictograph conservancy Welcome to the Spring 2009 edition of Wildlife Matters. As this edition goes to print, we are in the process of fi nalising the acquisition of Bowra (see pages 4-5), a 14,000 the awc mission hectare property located in the heart of the Mulga Lands in Queensland. Bowra will The mission of Australian Wildlife Conservancy be our 21st sanctuary, bringing the AWC network to more than 2.56 million hectares (AWC) is the effective conservation of all (6.3 million acres). Australian animal species and the habitats in While the overall scale of the portfolio is impressive, it is not the number of properties or which they live. To achieve this mission, our hectares that really count. A more accurate measure of the value of the portfolio is the actions are focused on: number of species and ecosystems that occur within the AWC estate. In this respect, • Establishing a network of sanctuaries the statistics are even more impressive – for example, around 80% of all Australian which protect threatened wildlife and terrestrial bird species and over 60% of all terrestrial mammal species occur on one or ecosystems: AWC now manages 20 more of our sanctuaries. sanctuaries covering over 2.56 million The fact that our portfolio captures such a high percentage of Australia’s wildlife species hectares (6.3 million acres). refl ects a deliberate, science-based strategy to ensure that AWC invests in properties • Implementing practical, on-ground of the highest environmental value. -

Terrestrial Native Mammals of Western Australia
TERRESTRIALNATIVE MAMMALS OF WESTERNAUSTRALIA On a number of occasionswe have been asked what D as y ce r cus u ist ica ud q-Mul Aara are the marsupialsof W.A. or what is the scientiflcname Anlechinusfla.t,ipes Matdo given to a palticular animal whosecommon name only A n t ec h i nus ap i ca I i s-Dlbbler rs known. Antechinusr osemondae-Little Red Antechinus As a guide,the following list of62 speciesof marsupials A nteclt itus mqcdonneIlens is-Red-eared Antechi nus and 59 speciesof othersis publishedbelow. Antechinus ? b ilar n i-Halney' s Antechinus Antec h in us mqculatrJ-Pismv Antechinus N ingaui r idei-Ride's Nirfaui - MARSUPALIA Ningauirinealvi Ealev's-KimNinsaui Ptaiigole*fuilissima beiiey Planigale Macropodidae Plani gale tenuirostris-Narrow-nosed Planigate Megaleia rufa Red Kangaroo Smi nt hopsis mu rina-Common Dulnart Macropus robustus-Etro Smin t hop[is longicaudat.t-Long-tailed Dunnart M acr opus fu Ii g inos,s-Western Grey Kangaroo Sminthops is cras sicaudat a-F at-tailed Dunnart Macrcpus antilo nus Antilope Kangaroo S-nint hopsi s froggal//- Larapinla Macropu"^agi /rs Sandy Wallaby Stnintllopsirgranuli,oer -Whire-railed Dunnart Macrcpus rirra Brush Wallaby Sninthopsis hir t ipes-Hairy -footed Dunnart M acro ptrs eugenii-T ammar Sminthopsiso oldea-^f r oughton's Dunnart Set oni x brac ltyuru s-Quokka A ntec h inomys lanrger-Wuhl-Wuhl On y ch oga I ea Lng uife r a-Kar r abul M.yr nte c o b ius fasc ialrls-N umbat Ony c hogalea Iunq ta-W \rrur.g Notoryctidae Lagorchest es conspic i Ilat us,Spectacied Hare-Wallaby Notorlctes -

Mulgara Husbandry Ma
Husbandry Guidelines Mulgara (Dasycercus cristicauda) Alice Springs Desert Park 2007 Compiled by Wes Caton CONTENTS: TAXONOMY.....................................................................................................................1 Common Name................................................................................................1.1 Classifacation...................................................................................................1.2 A.S.M.P. Category...........................................................................................1.3 I.U.C.N. Category............................................................................................1.4 O.H.&S. Category...........................................................................................1.5 Studbook Keeper.............................................................................................1.6 NATURAL HISTORY......................................................................................................2 Family..............................................................................................................2.1 DISTRIBUTION...............................................................................................................3 Map...................................................................................................................3.1 Habitat .............................................................................................................3.2 MORPHOMETRICS........................................................................................................4 -

Ecology and Predator Associations of the Northern Quoll (Dasyurus Hallucatus) in the Pilbara Lorna Hernandez Santin M.Sc
Ecology and predator associations of the northern quoll (Dasyurus hallucatus) in the Pilbara Lorna Hernandez Santin M.Sc. A thesis submitted for the degree of Doctor of Philosophy at The University of Queensland in 2016 School of Biological Sciences Abstract The northern quoll (Dasyurus hallucatus) is an endangered carnivorous marsupial in the family Dasyuridae that occurs in the northern third of Australia. It has declined throughout its range, especially in open, lowland habitats. This led to the hypothesis that rocky outcrops where it persists provide a safe haven from introduced predators and provide greater microhabitat heterogeneity associated with higher prey availability. Proposed causes of decline include introduced cane toads (Rhinella marina, which are toxic), predation by feral cats (Felis catus), foxes (Vulpes vulpes), and the dingo (Canis lupus), and habitat alteration by changes in fire regimes. The National Recovery Plan highlighted knowledge gaps relevant to the conservation of the northern quoll. These include assembling data on ecology and population status, determining factors in survival, especially introduced predators, selecting areas that can be used as refuges, and identifying and securing key populations. The Pilbara region in Western Australia is a key population currently free of invasive cane toads (a major threat). The Pilbara is a semi-arid to arid area subject to cyclones between December and March. I selected two sites: Millstream Chichester National Park (2 381 km2) and Indee Station (1 623 km2). These are dominated by spinifex (hummock) grasslands, with rugged rock outcrops, shrublands, riparian areas, and some soft (tussock) grasslands. They are subject to frequent seasonal fires, creating a mosaic of recently burnt and longer unburnt areas. -

Skulls of Tasmania
SKULLS of the MAMMALS inTASMANIA R.H.GREEN with illustrations by 1. L. RAINBIRIJ An Illustrated Key to the Skulls of the Mammals in Tasmania by R. H. GREEN with illustrations by J. L. RAINBIRD Queen Victoria Museum and Art Gallery, Launceston, Tasmania Published by Queen Victoria Museum and Art Gallery, Launceston, Tasmania, Australia 1983 © Printed by Foot and Playsted Pty. Ltd., Launceston ISBN a 7246 1127 4 2 CONTENTS Page Introduction . 4 Acknowledgements.......................... 5 Types of teeth........................................................................................... 6 The illustrations........................................ 7 Skull of a carnivore showing polyprotodont dentition 8 Skull of a herbivore showing diprotodont dentition......................................... 9 Families of monotremes TACHYGLOSSIDAE - Echidna 10 ORNITHORHYNCHIDAE - Platypus 12 Families of marsupials DASYURIDAE - Quolls, devil, antechinuses, dunnart 14 THYLACINIDAE - Thylacine 22 PERAMELIDAE - Bandicoots 24 PHALANGERIDAE - Brushtail Possum 28 BURRAMYIDAE - Pygmy-possums 30 PETAURIDAE - Sugar glider, ringtail 34 MACROPODIDAE - Bettong, potoroo, pademelon, wallaby, kangaroo 38 VOMBATIDAE - Wombat 44 Families of eutherians VESPERTILIONIDAE - Bats 46 MURIDAE - Rats, mice 56 CANIDAE - Dog 66 FELIDAE - Cat 68 EQUIDAE - Horse 70 BOVIDAE - Cattle, goat, sheep 72 CERVIDAE - Deer 76 SUIDAE - Pig 78 LEPORIDAE - Hare, rabbit 80 OTARIIDAE - Sea-lion, fur-seals 84 PHOCIDAE - Seals 88 HOMINIDAE - Man 92 Appendix I Dichotomous key 94 Appendix II Index to skull illustrations . ........... 96 Alphabetical index of common names . ........................................... 98 Alphabetical index of scientific names 99 3 INTRODUCTION The skulls of mammals are often brought to museums for indentification. The enquirers may be familiar with the live animal but they are often quite confused when confronted with the task of identifying a skull or, worse, only part of a skull. Skulls may be found in the bush with, or apart from, the rest of the skeleton. -
Supporting Information
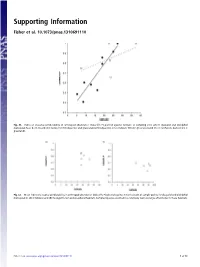
Supporting Information Fisher et al. 10.1073/pnas.1310691110 Fig. S1. Index of seasonal predictability in arthropod abundance (Colwell’s P) plotted against latitude of sampling sites where dasyurid and didelphid marsupials have been recorded in rainforest (filled points) and grassland (unfilled points). Lines indicate fitted regressions (solid line = rainforest, dashed line = grassland). Fig. S2. Mean index of seasonal predictability in arthropod abundance (Colwell’s P) plotted against mean latitude of sample points for dasyurid and didelphid marsupials in (A) shrubland and (B) Eucalypt forest and woodland habitats. Sampled species occurred in a relatively narrow range of latitudes in these habitats. Fisher et al. www.pnas.org/cgi/content/short/1310691110 1of13 Fig. S3. Phylogeny of insectivorous marsupials with known life history data, based on ref. 1 with updates from ref. 2. 1. Cardillo M, Bininda-Emonds ORP, Boakes E, Purvis A (2004) A species-level phylogenetic supertree of marsupials. J Zool 264(1):11–31. 2. Fritz SA, Bininda-Emonds ORP, Purvis A (2009) Geographical variation in predictors of mammalian extinction risk: Big is bad, but only in the tropics. Ecol Lett 12(6):538–549. Fisher et al. www.pnas.org/cgi/content/short/1310691110 2of13 Fisher et al. Table S1. Reproductive traits and diets of insectivorous marsupials Latitude (south) www.pnas.org/cgi/content/short/1310691110 Proportion Proportion Female for Habitat of of age species class for females males Male Breeding Copulation Litters Scrotal at first with species Genus species