Effects of a Landscape Disturbance on the Habitat Use and Behavior of the Black Racer
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Checklist of Reptiles and Amphibians Revoct2017
CHECKLIST of AMPHIBIANS and REPTILES of ARCHBOLD BIOLOGICAL STATION, the RESERVE, and BUCK ISLAND RANCH, Highlands County, Florida. Voucher specimens of species recorded from the Station are deposited in the Station reference collections and the herpetology collection of the American Museum of Natural History. Occurrence3 Scientific name1 Common name Status2 Exotic Station Reserve Ranch AMPHIBIANS Order Anura Family Bufonidae Anaxyrus quercicus Oak Toad X X X Anaxyrus terrestris Southern Toad X X X Rhinella marina Cane Toad ■ X Family Hylidae Acris gryllus dorsalis Florida Cricket Frog X X X Hyla cinerea Green Treefrog X X X Hyla femoralis Pine Woods Treefrog X X X Hyla gratiosa Barking Treefrog X X X Hyla squirella Squirrel Treefrog X X X Osteopilus septentrionalis Cuban Treefrog ■ X X Pseudacris nigrita Southern Chorus Frog X X Pseudacris ocularis Little Grass Frog X X X Family Leptodactylidae Eleutherodactylus planirostris Greenhouse Frog ■ X X X Family Microhylidae Gastrophryne carolinensis Eastern Narrow-mouthed Toad X X X Family Ranidae Lithobates capito Gopher Frog X X X Lithobates catesbeianus American Bullfrog ? 4 X X Lithobates grylio Pig Frog X X X Lithobates sphenocephalus sphenocephalus Florida Leopard Frog X X X Order Caudata Family Amphiumidae Amphiuma means Two-toed Amphiuma X X X Family Plethodontidae Eurycea quadridigitata Dwarf Salamander X Family Salamandridae Notophthalmus viridescens piaropicola Peninsula Newt X X Family Sirenidae Pseudobranchus axanthus axanthus Narrow-striped Dwarf Siren X Pseudobranchus striatus -

Snakes of the Everglades Agricultural Area1 Michelle L
CIR1462 Snakes of the Everglades Agricultural Area1 Michelle L. Casler, Elise V. Pearlstine, Frank J. Mazzotti, and Kenneth L. Krysko2 Background snakes are often escapees or are released deliberately and illegally by owners who can no longer care for them. Snakes are members of the vertebrate order Squamata However, there has been no documentation of these snakes (suborder Serpentes) and are most closely related to lizards breeding in the EAA (Tennant 1997). (suborder Sauria). All snakes are legless and have elongated trunks. They can be found in a variety of habitats and are able to climb trees; swim through streams, lakes, or oceans; Benefits of Snakes and move across sand or through leaf litter in a forest. Snakes are an important part of the environment and play Often secretive, they rely on scent rather than vision for a role in keeping the balance of nature. They aid in the social and predatory behaviors. A snake’s skull is highly control of rodents and invertebrates. Also, some snakes modified and has a great degree of flexibility, called cranial prey on other snakes. The Florida kingsnake (Lampropeltis kinesis, that allows it to swallow prey much larger than its getula floridana), for example, prefers snakes as prey and head. will even eat venomous species. Snakes also provide a food source for other animals such as birds and alligators. Of the 45 snake species (70 subspecies) that occur through- out Florida, 23 may be found in the Everglades Agricultural Snake Conservation Area (EAA). Of the 23, only four are venomous. The venomous species that may occur in the EAA are the coral Loss of habitat is the most significant problem facing many snake (Micrurus fulvius fulvius), Florida cottonmouth wildlife species in Florida, snakes included. -

A Guide to Missouri's Snakes
A GUIDE TO MISSOURI’S SNAKES MISSOURI DEPARTMENT OF CONSERVATION A Guide to Missouri’s Snakes by Jeffrey T. Briggler, herpetologist, and Tom R. Johnson, retired herpetologist, Missouri Department of Conservation Photographs by Jeffrey T. Briggler, Richard Daniel, Tom R. Johnson, and Jim Rathert Edited by Larry Archer Design by Susan Ferber Front cover: Eastern milksnake. Photo by Jim Rathert. mdc.mo.gov Copyright © 2017 by the Conservation Commission of the State of Missouri Published by the Missouri Department of Conservation PO Box 180, Jefferson City, Missouri 65102–0180 Equal opportunity to participate in and benefit from programs of the Missouri Depart- ment of Conservation is available to all individuals without regard to their race, color, religion, national origin, sex, ancestry, age, sexual orientation, veteran status, or disability. Questions should be directed to the Department of Conser- vation, PO Box 180, Jefferson City, MO 65102, 573-751-4115 (voice) or 800-735-2966 (TTY), or to Chief, Public Civil Rights, Office of Civil Rights, U.S. Department of the Interior, 1849 C Street, NW, Washington, D.C. 20240. GET TO KNOW MISSOURI’S SNAKES Snakes have generated more fear and misunderstanding than any other group of animals. Psychologists have proven that a fear of snakes (called ophidiophobia) is acquired; we are not born with it. Once people learn some of the interesting facts about snakes and discover that most of them are harmless and beneficial, their aversion may diminish. With patience and understanding, almost anyone can overcome a dread of snakes and actually enjoy studying them. One thing is certain — even people with a well-developed fear of snakes are curious about them. -

Venomous Nonvenomous Snakes of Florida
Venomous and nonvenomous Snakes of Florida PHOTOGRAPHS BY KEVIN ENGE Top to bottom: Black swamp snake; Eastern garter snake; Eastern mud snake; Eastern kingsnake Florida is home to more snakes than any other state in the Southeast – 44 native species and three nonnative species. Since only six species are venomous, and two of those reside only in the northern part of the state, any snake you encounter will most likely be nonvenomous. Florida Fish and Wildlife Conservation Commission MyFWC.com Florida has an abundance of wildlife, Snakes flick their forked tongues to “taste” their surroundings. The tongue of this yellow rat snake including a wide variety of reptiles. takes particles from the air into the Jacobson’s This state has more snakes than organs in the roof of its mouth for identification. any other state in the Southeast – 44 native species and three nonnative species. They are found in every Fhabitat from coastal mangroves and salt marshes to freshwater wetlands and dry uplands. Some species even thrive in residential areas. Anyone in Florida might see a snake wherever they live or travel. Many people are frightened of or repulsed by snakes because of super- stition or folklore. In reality, snakes play an interesting and vital role K in Florida’s complex ecology. Many ENNETH L. species help reduce the populations of rodents and other pests. K Since only six of Florida’s resident RYSKO snake species are venomous and two of them reside only in the northern and reflective and are frequently iri- part of the state, any snake you en- descent. -

2011 USFWS North Florida ESO Eastern Indigo Snake Survey Protocol
Survey Protocol for the Eastern Indigo Snake, Drymarchon couperi, in North and Central Florida September 2011 Photo: FWS Purpose and Scope The purpose of this document is to provide a tool to improve the review of permit applications and proposed land clearing activities for potential effects on the federally-threatened eastern indigo snake (indigo snake), in accordance with Endangered Species Act of 1973 (ESA), as amended (16 U.S.C. 1531 et seq.). The tool is applicable to the U.S. Fish and Wildlife Service (Service) North Florida Ecological Services Field Office (NFESFO) geographic area of responsibility, which includes the following counties: Alachua, Baker, Bradford, Brevard, Citrus, Clay, Columbia, Dixie, Duval, Flagler, Gilchrist, Hamilton, Hernando, Hillsborough, Lafayette, Lake, Levy, Madison, Manatee, Marion, Nassau, Orange, Pasco, Pinellas, Putnam, St. Johns, Seminole, Sumter, Suwannee, Taylor, Union, and Volusia. The tool is a visual encounter survey protocol (Protocol) that is to be conducted by project proponents or their designees for determining the presence of indigo snakes at a proposed project site. The results of the Protocol will be used by Federal and non-Federal entities in evaluating permit applications and proposed activities for compliance with the ESA. The Protocol is primarily designed to be used by the U.S. Army Corps of Engineers in conjunction with their Eastern Indigo Snake Programmatic Effect Determination Key. However, the NFESFO encourages the use of the Protocol by other project proponents, and Federal and non-Federal permitting entities in situations where habitats that may support indigo snakes will be impacted by development activities. For projects that encompass vast acreage such as Developments of Regional Impact, military installations, and large restoration projects, we recommend contacting our office in advance to discuss the best approach for implementation of this Protocol in order to achieve the intended purpose and objective. -
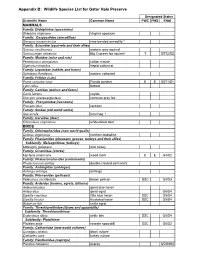
GHP Wildlife List
Appendix B: Wildlife Species List for Gator Hole Preserve Designated Status Scientific Name Common Name FWC FWS FNAI MAMMALS Family: Didelphidae (opossums) Didelphis virginiana Virginia opossum Family: Dasypodidae (armadillos) Dasypus novemcinctus nine-banded armadillo * Family: Sciuridae (squirrels and their allies) Sciurus carolinensis eastern gray squirrel Sciurus niger avicennia Big Cypress fox squirrel T G5T2/S2 Family: Muridae (mice and rats) Peromyscus gossypinus cotton mouse Sigmodon hispidus hispid cotton rat Family: Leporidae (rabbits and hares) Sylvilagus floridanus eastern cottontail Family: Felidae (cats) Puma concolor coryi Florida panther E E G5T1/S1 Lynx rufus bobcat Family: Canidae (wolves and foxes) Canis latrans coyote Urocyon cinereoargenteus common gray fox Family: Procyonidae (raccoons) Procyon lotor raccoon Family: Suidae (old world swine) Sus scrofa feral hog * Family: Cervidae (deer) Odocoileus virginianus white-tailed deer BIRDS Family: Odontophoridae (new world quails) Colinus virginianus northern bobwhite Family: Phasianidae (pheasant, grouse, turkeys and their allies) Subfamily: Meleagridinae (turkeys) Meleagris gallopavo wild turkey Family: Ciconiidae (storks) Mycteria americana wood stork E E G4/S2 Family: Phalacrocoracidae (cormorants) Phalacrocorax auritus double-crested cormorant Family: Anhingidae (anhingas) Anhinga anhinga anhinga Family: Pelecanidae (pelicans) Pelecanus occidentalis brown pelican SSC G4/S3 Family: Ardeidae (herons, egrets, bitterns) Ardea herodius great blue heron Ardea alba great egret -

Appendix C: Species Lists
Appendix C: Species Lists Appendix C: Species Lists List of Bird Species / page 99 List of Mammal Species / page 106 List of Herptofauna Species / page 107 List of Fish Species / page 109 List of Fresh Water Mussel Species / page 112 List of Plant Species / page 113 Patoka River National Wildlife Refuge/Management Area / Comprehensive Conservation Plan 97 Appendix C: Species Lists Appendix C: Species Lists Bird Species List, Patoka River NWR & MA Common Name Scientific Name Patoka Cane Noted in Indiana River Ridge Comprehensive WMA Wildlife Strategy as Species of Greatest Conservation Need Common loon Gavia immer ✔ Pied-billed grebe Podilymbus podiceps ✔ ✔ Horned grebe Podiceps auritus ✔ American white pelican Pelecanus erythrorhynchos ✔ Double-crested cormorant Phalacrocorax auritus ✔ ✔ American bittern Botaurus lentiginosus ✔ ✔ ✔ Least bittern Ixobrychus exilis ✔ ✔ ✔ Great blue heron Ardea herodias ✔ ✔ Snowy egret Egretta thula ✔ ✔ Great egret Casmerodius albus ✔ ✔ ✔ Little blue heron Egretta caerulea ✔ ✔ Cattle egret Bubulcus ibis ✔ ✔ Green heron Butorides striatus ✔ ✔ Black-crowned night-heron Nycticorax nycticorax ✔ ✔ ✔ Yellow-crowned night-heron Nycticorax violaceus ✔ ✔ White-faced ibis Plegadis chihi ✔ Wood stork Mycteria americana ✔ Greater white-fronted goose Anser albifrons ✔ ✔ Snow goose Chen caerulescens ✔ ✔ Canada goose Branta canadensis ✔ ✔ Tundra swan Cygnus columbianus ✔ ✔ Trumpeter swan Cygnus buccinator ✔ ✔ Mute swan Cygnus olor ✔ Wood duck Aix sponsa ✔ ✔ Gadwall Anas strepera ✔ ✔ American wigeon Anas americana ✔ ✔ American -

Final Environmental Impact Statement(Whole Document)
FINAL ENVIRONMENTAL IMPACT STATEMENT United States Land and Resource Management Plan Department of for the Daniel Boone National Forest Agriculture Forest Service Southern Region Daniel Boone National Forest Winchester, KY April 2004 Management Bulletin R8-MB 117B Nondiscrimination Notice The U.S. Department of Agriculture Forest Service prohibits discrimination in all its programs and activities on the basis of race, color, national origin, sex, religion, age, disability, political beliefs, sexual orientation, and marital or family status (not all prohibited bases apply to all programs). Persons with disabilities who require alternative means for communication of program information (Braille, large print, audiotape, etc.) should contact USDA's TARGET Center at (202) 720-2600 (voice and TDD). To file a complaint of discrimination write: USDA, Director, Office of Civil Rights, Room 326-W, Whitten Building, 14th and Independence Ave, SW, Washington, DC 20250-9410 or call (202) 720- 5964 (voice or TDD). USDA Forest Service is an equal opportunity provider and employer. Disclaimer of Non-endorsement Reference in this document to any specific commercial products, process, or service by trade name, trademark, manufacturer, or otherwise, does not necessarily constitute or imply its endorsement, recommendation, or favoring by the United States Government. Disclaimer for External Links The appearance of hyperlinks in this document does not constitute endorsement by the Department of Agriculture of the linked web sites, or the information, products -

Northern Black Racer Species Assessment
NORTHERN BLACK RACER ASSESSMENT FINAL July 19, 2007 Prepared by: Jonathan D. Mays and Charlie S. Todd Maine Department of Inland Fisheries and Wildlife Wildlife Division Resource Assessment Section NORTHERN BLACK RACER ASSESSMENT TABLE OF CONTENTS Page INTRODUCTION...........................................................................................................4 NATURAL HISTORY.....................................................................................................5 Description..........................................................................................................5 Distribution..........................................................................................................7 Taxonomy...........................................................................................................8 Habitat ................................................................................................................9 Activity and Movements....................................................................................11 Diet ...................................................................................................................13 Reproductive Ecology.......................................................................................14 Survival, Growth, and Longevity.......................................................................15 MANAGEMENT ..........................................................................................................17 Regulatory Authority .........................................................................................17 -
St. Marks National Wildlife Refuge Amphibian, Reptile and Mammal List
U.S. Fish & Wildlife Service St. Marks National Wildlife Refuge Amphibian, Reptile and Mammal List alligator - Tom Darragh deer: Joe Bonislawsky This blue goose, designed by J.N. “Ding” Darling, has become a symbol of the National Wildlife Refuge System. gopher tortoise - Pierson Hill The St. Marks National Wildlife Refuge was established in 1931 and today encompasses 70,000 acres. It’s wide diversity of habitats, including open water, salt marsh, swamps, freshwater pools, hardwoods, and upland pine areas make the refuge home for an equally wide variety of wildlife. The St. Marks NWR provides nesting habitat for these Federal and squirrel tree frog: Pierson Hill State endangered and threatened birds: the Southern bald eagle, least tern, and red-cockaded woodpecker. Other endangered or rare species include the woodstork, swallow-tailed kite, peregrine falcon, American alligator, Eastern indigo snake and the Florida black bear. Visitors may also observe loggerhead sea turtles and West Indian manatees offshore from the lighthouse. Many state-listed threatened and endangered plants are also found on the refuge. eastern coachwhip: Mike Keys The following list contains the 38 species of amphibians, 69 species of reptiles, and 44 species of mammals compiled from observations, consultation with experts in respective fields, and literature research. Some species are more common seasonally and some are nocturnal. Look for evidence such as tracks, burrows, grass tunnels, and other signs of activity. Careful eyes and attentive ears can uncover numerous -

Animal Checklist Volusia County, Florida
Page 1 of 2 Lake George Preserve Animal Checklist Volusia County, Florida Amphibian Bird (continued) Ranidae Southern Leopard Frog Lithobates sphenocephalus Corvidae American Crow Corvus brachyrhynchos Corvidae Blue Jay Cyanocitta cristata Arachnid Corvidae Fish Crow Corvus ossifragus Salticidae Regal Jumping Spider Phidippus regius Dromaiidae Emu Dromaius novaehollandiae Bird Accipitridae Bald Eagle Haliaeetus leucocephalus Emberizidae Bachman's Sparrow Aimophila aestivalis Accipitridae Broad-winged Hawk Buteo platypterus Emberizidae Eastern Towhee Pipilo erythrophthalmus Accipitridae Red-shouldered Hawk Buteo lineatus Accipitridae Swallow Tailed Kite Elanoides forficatus Gruidae Sandhill Crane Grus canadensis Alcedinidae Belted Kingfisher Megaceryle alcyon Icteridae Boat-tailed Grackle Quiscalus major Anatidae Wood Duck Aix sponsa Icteridae Common Grackle Quiscalus quiscula Icteridae Red-winged Blackbird Agelaius phoeniceus Anhingidae Anhinga Anhinga anhinga Laridae Caspian Tern Sterna caspia Apodidae Chimney Swift Chaetura pelagica Laridae Forster's Tern Sterna forsteri Ardeidae Cattle Egret Bubulcus ibis Laridae Laughing Gull Leucophaeus atricilla Ardeidae Great Blue Heron Ardea herodias Laridae Royal Tern Sterna maxima Ardeidae Great Egret Casmerodius albus Ardeidae Little Blue Heron Egretta caerulea Odontophoridae Northern Bobwhite Colinus virginianus Ardeidae Snowy Egret Egretta thula Ardeidae Tricolored Heron Egretta tricolor Pandionidae Osprey Pandion haliaetus Caprimulgidae Chuck-will's-widow Caprimulgus carolinensis Paridae -

001966 SDEIS Appendices
FINAL ENVIRONMENTAL IMPACT STATEMENT SPRINGDALE NORTHERN BYPASS APPENDIX I TERRESTRIAL AND AQUATIC FAUNA FINAL ENVIRONMENTAL IMPACT STATEMENT SPRINGDALE NORTHERN BYPASS BIRDS Common Name Scientific Name Typical Habitat Seasonal Status Order Podicipediformes Pied-billed Grebe Podilymbus podiceps Still water P Horned Grebe Podiceps auritus Still water W Western Grebe Aechmophorus occidentalis Still water T Order Pelecaniformes American White Pelican Pelecanus erythrorhynchos Open water T Double-crested Cormorant Phalacrocorax auritus Open water W Magnificent Frigatebird Fregata magnificers Open water T Order Ciconiiformes American Bittern Botunrus lentiginosus Marshes T Least Bittern Ixobrychus exilis Marshes T Great Blue Heron Ardea herodias Still water P Great Egret Casmerodius albus Still water S Snowy Egret Egretta thula Still water S Little Blue Heron Egretta caerulea Still water S Tricolored Heron Egretta tricolor Still water T Cattle Egret Bubulcus ibis Open fields S Green-backed Heron Butorides striatus Still water S Black-crowned Night Heron Nycticorax nycticorax Still water S Yellow-crowned Night Heron Nyctanassa violaceus Still water S White-faced Ibis Plegadis chihi Open Water T Order Anseriformes Greater White-fronted Goose Anser albifrons Open fields W Snow Goose Chen caerulescens Open fields T Ross’ Goose Chen rossii Still water T Canada Goose Branta canadensis Open fields W Wood Duck Aix sponsa Swamps P Green-winged Teal Anas crecca Open water W Mallard Anas platyrhynchos Still water W Northern Pintail Anas acuta Still water