Patronus Is the Elusive Plant Securin, Preventing Chromosome Separation by Antagonizing Separase
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Serine Proteases with Altered Sensitivity to Activity-Modulating
(19) & (11) EP 2 045 321 A2 (12) EUROPEAN PATENT APPLICATION (43) Date of publication: (51) Int Cl.: 08.04.2009 Bulletin 2009/15 C12N 9/00 (2006.01) C12N 15/00 (2006.01) C12Q 1/37 (2006.01) (21) Application number: 09150549.5 (22) Date of filing: 26.05.2006 (84) Designated Contracting States: • Haupts, Ulrich AT BE BG CH CY CZ DE DK EE ES FI FR GB GR 51519 Odenthal (DE) HU IE IS IT LI LT LU LV MC NL PL PT RO SE SI • Coco, Wayne SK TR 50737 Köln (DE) •Tebbe, Jan (30) Priority: 27.05.2005 EP 05104543 50733 Köln (DE) • Votsmeier, Christian (62) Document number(s) of the earlier application(s) in 50259 Pulheim (DE) accordance with Art. 76 EPC: • Scheidig, Andreas 06763303.2 / 1 883 696 50823 Köln (DE) (71) Applicant: Direvo Biotech AG (74) Representative: von Kreisler Selting Werner 50829 Köln (DE) Patentanwälte P.O. Box 10 22 41 (72) Inventors: 50462 Köln (DE) • Koltermann, André 82057 Icking (DE) Remarks: • Kettling, Ulrich This application was filed on 14-01-2009 as a 81477 München (DE) divisional application to the application mentioned under INID code 62. (54) Serine proteases with altered sensitivity to activity-modulating substances (57) The present invention provides variants of ser- screening of the library in the presence of one or several ine proteases of the S1 class with altered sensitivity to activity-modulating substances, selection of variants with one or more activity-modulating substances. A method altered sensitivity to one or several activity-modulating for the generation of such proteases is disclosed, com- substances and isolation of those polynucleotide se- prising the provision of a protease library encoding poly- quences that encode for the selected variants. -

Separase Protease Activity Is Required for Cytokinesis in Addition
bioRxiv preprint doi: https://doi.org/10.1101/069906; this version posted August 16, 2016. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. Separase Protease Activity is Required for Cytokinesis in addition to Chromosome Segregation Xiaofei Bai1 and Joshua N. Bembenek1* 1Department of Biochemistry, Cellular and Molecular Biology, University of Tennessee, Knoxville, Tennessee, United States of America * Corresponding Author: Joshua N. Bembenek 1414 Cumberland Ave. C211 Walters Life Sciences Building Knoxville, TN 37996 (865)-974-4085 E-mail: [email protected] bioRxiv preprint doi: https://doi.org/10.1101/069906; this version posted August 16, 2016. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. Abstract Chromosomal segregation and cytokinesis are tightly regulated processes required for successful cell division. The cysteine protease separase cleaves a subunit of the cohesin complex to allow chromosome segregation at anaphase onset. Separase also regulates meiotic cortical granule exocytosis and vesicle trafficking during cytokinesis, both of which involve RAB-11. Separase has non-proteolytic signaling functions in addition to its role in substrate cleavage, and its mechanism in exocytosis is unknown. We sought to determine whether separase regulates RAB-11 vesicle exocytosis through a proteolytic or non-proteolytic mechanism. -

Proteolytic Enzymes in Grass Pollen and Their Relationship to Allergenic Proteins
Proteolytic Enzymes in Grass Pollen and their Relationship to Allergenic Proteins By Rohit G. Saldanha A thesis submitted in fulfilment of the requirements for the degree of Masters by Research Faculty of Medicine The University of New South Wales March 2005 TABLE OF CONTENTS TABLE OF CONTENTS 1 LIST OF FIGURES 6 LIST OF TABLES 8 LIST OF TABLES 8 ABBREVIATIONS 8 ACKNOWLEDGEMENTS 11 PUBLISHED WORK FROM THIS THESIS 12 ABSTRACT 13 1. ASTHMA AND SENSITISATION IN ALLERGIC DISEASES 14 1.1 Defining Asthma and its Clinical Presentation 14 1.2 Inflammatory Responses in Asthma 15 1.2.1 The Early Phase Response 15 1.2.2 The Late Phase Reaction 16 1.3 Effects of Airway Inflammation 16 1.3.1 Respiratory Epithelium 16 1.3.2 Airway Remodelling 17 1.4 Classification of Asthma 18 1.4.1 Extrinsic Asthma 19 1.4.2 Intrinsic Asthma 19 1.5 Prevalence of Asthma 20 1.6 Immunological Sensitisation 22 1.7 Antigen Presentation and development of T cell Responses. 22 1.8 Factors Influencing T cell Activation Responses 25 1.8.1 Co-Stimulatory Interactions 25 1.8.2 Cognate Cellular Interactions 26 1.8.3 Soluble Pro-inflammatory Factors 26 1.9 Intracellular Signalling Mechanisms Regulating T cell Differentiation 30 2 POLLEN ALLERGENS AND THEIR RELATIONSHIP TO PROTEOLYTIC ENZYMES 33 1 2.1 The Role of Pollen Allergens in Asthma 33 2.2 Environmental Factors influencing Pollen Exposure 33 2.3 Classification of Pollen Sources 35 2.3.1 Taxonomy of Pollen Sources 35 2.3.2 Cross-Reactivity between different Pollen Allergens 40 2.4 Classification of Pollen Allergens 41 2.4.1 -

Patronus Is the Elusive Plant Securin, Preventing Chromosome Separation By
bioRxiv preprint doi: https://doi.org/10.1101/606285; this version posted April 11, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. 1 Patronus is the elusive plant securin, preventing chromosome separation by 2 antagonizing separase 3 Laurence Cromer1, Sylvie Jolivet1, Dipesh Kumar Singh1, Floriane Berthier1, Nancy 4 De Winne2, Geert De Jaeger2, Shinichiro Komaki3,4, Maria Ada Prusicki3, Arp 5 Schnittger3, Raphael Guérois5 and Raphael Mercier1* 6 7 1 Institut Jean-Pierre Bourgin, UMR1318 INRA-AgroParisTech, Université Paris- 8 Saclay, RD10, 78000 Versailles, France. 9 2 Department of Plant Biotechnology and Bioinformatics, Ghent University, Ghent, 10 Belgium, VIB Center for Plant Systems Biology, Ghent, Belgium 11 3 Department of Developmental Biology, Institute for Plant Sciences and 12 Microbiology, University of Hamburg, 22609 Hamburg, Germany. 13 4 Present address : Nara Institute of Science and Technology, Graduate School of 14 Biological Sciences, 8916-5 Takayama, Ikoma, Nara 630-0192, Japan 15 5 Institute for Integrative Biology of the Cell (I2BC), Commissariat à l’Energie 16 Atomique et aux Energies Alternatives (CEA), Centre National de la Recherche 17 Scientifique (CNRS), Université Paris-Sud, CEA-Saclay, Gif-sur-Yvette, France 18 * Corresponding author. [email protected] 19 1 bioRxiv preprint doi: https://doi.org/10.1101/606285; this version posted April 11, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. -
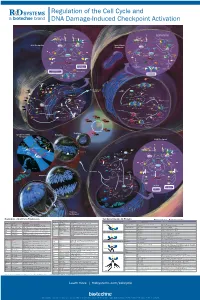
Regulation of the Cell Cycle and DNA Damage-Induced Checkpoint Activation
RnDSy-lu-2945 Regulation of the Cell Cycle and DNA Damage-Induced Checkpoint Activation IR UV IR Stalled Replication Forks/ BRCA1 Rad50 Long Stretches of ss-DNA Rad50 Mre11 BRCA1 Nbs1 Rad9-Rad1-Hus1 Mre11 RPA MDC1 γ-H2AX DNA Pol α/Primase RFC2-5 MDC1 Nbs1 53BP1 MCM2-7 53BP1 γ-H2AX Rad17 Claspin MCM10 Rad9-Rad1-Hus1 TopBP1 CDC45 G1/S Checkpoint Intra-S-Phase RFC2-5 ATM ATR TopBP1 Rad17 ATRIP ATM Checkpoint Claspin Chk2 Chk1 Chk2 Chk1 ATR Rad50 ATRIP Mre11 FANCD2 Ubiquitin MDM2 MDM2 Nbs1 CDC25A Rad50 Mre11 BRCA1 Ub-mediated Phosphatase p53 CDC25A Ubiquitin p53 FANCD2 Phosphatase Degradation Nbs1 p53 p53 CDK2 p21 p21 BRCA1 Ub-mediated SMC1 Degradation Cyclin E/A SMC1 CDK2 Slow S Phase CDC45 Progression p21 DNA Pol α/Primase Slow S Phase p21 Cyclin E Progression Maintenance of Inhibition of New CDC6 CDT1 CDC45 G1/S Arrest Origin Firing ORC MCM2-7 MCM2-7 Recovery of Stalled Replication Forks Inhibition of MCM10 MCM10 Replication Origin Firing DNA Pol α/Primase ORI CDC6 CDT1 MCM2-7 ORC S Phase Delay MCM2-7 MCM10 MCM10 ORI Geminin EGF EGF R GAB-1 CDC6 CDT1 ORC MCM2-7 PI 3-Kinase p70 S6K MCM2-7 S6 Protein Translation Pre-RC (G1) GAB-2 MCM10 GSK-3 TSC1/2 MCM10 ORI PIP2 TOR Promotes Replication CAK EGF Origin Firing Origin PIP3 Activation CDK2 EGF R Akt CDC25A PDK-1 Phosphatase Cyclin E/A SHIP CIP/KIP (p21, p27, p57) (Active) PLCγ PP2A (Active) PTEN CDC45 PIP2 CAK Unwinding RPA CDC7 CDK2 IP3 DAG (Active) Positive DBF4 α Feedback CDC25A DNA Pol /Primase Cyclin E Loop Phosphatase PKC ORC RAS CDK4/6 CDK2 (Active) Cyclin E MCM10 CDC45 RPA IP Receptor -

Control of the Cell Cycle During Meiotic Maturation of Mouse Oocytes
Control of the cell cycle during meiotic maturation of mouse oocytes Ibtissem Nabti Department of Cell and Developmental Biology University College London London, UK A thesis submitted for the degree of Doctor of Philosophy April 2010 Declaration The work presented in this thesis was conducted under the supervision of Professor John Carroll in the department of cell and developmental biology, University College London, and Professor Keith Jones in the department of reproductive physiology, University of Newcastle upon Tyne. I, Ibtissem Nabti confirm that the data presented in this thesis is my own and from original studies. Also, this dissertation has not previously been submitted, wholly or in part, to any other university for any degree or diploma. Ibtissem Nabti 1 Abstract The overall aim of the work presented in this thesis is to better understand the molecular mechanisms underlying the progression of the meiotic division in mammalian oocytes. The first goal of this project was to investigate the relative contribution of securin and cyclin B1 to the control of the protease separase during the indeterminate period of metaphase II arrest. I found here that although there are conditions in which either securin or MPF can prevent chromosome disjunction, such as during meiosis I, securin is the predominant inhibitor of separase during metaphase II arrest. Thus, CDK1 pharmacological inhibition as well as antibody inhibition of CDK1/cyclin B1 binding to separase, both failed to induce sister chromatid disjunction. Instead, securin morpholino knockdown induced sister chromatid separation, which could be rescued by injection of securin cRNA. I also examined the effect of CDK1 and MAPK activities on securin stability. -

Developmental Requirements for Ubiquitin-Mediated Proteolysis
REVIEW 773 Development 133, 773-784 doi:10.1242/dev.02276 Degrade to create: developmental requirements for ubiquitin-mediated proteolysis during early C. elegans embryogenesis Bruce Bowerman1 and Thimo Kurz2,* The ubiquitin protein conjugation system tags proteins with the the activity of cell division kinases, which must bind to cyclin small polypeptide ubiquitin. Most poly-ubiquitinated proteins proteins to be active (Morgan, 1997; Murray, 2004). Cyclins were are recognized and degraded by the proteasome, a large multi- so named because they accumulate to peak levels at specific times subunit protease. Ubiquitin-dependent protein degradation is during the cell cycle, thereby activating cell division kinases at used as a regulatory tool for many essential processes, the best appropriate moments. A regulatory role for ubiquitin in the cell cycle studied of which is eukaryotic cell cycle progression. More was discovered when it was shown that cyclins are targeted for rapid recently, genetic studies in C. elegans have identified multiple degradation during the cell cycle by poly-ubiquitination (Glotzer et roles for the ubiquitin system in early development, where al., 1991; Hershko et al., 1991). ubiquitin-dependent protein degradation governs such diverse Additional roles for ubiquitination emerged from studies of the events as passage through meiosis, cytoskeletal regulation and metaphase-to-anaphase transition during mitosis (Peters, 2002). cell fate determination. Early in mitosis, the duplicated chromosomes (sister chromatids) condense and are held together by a multi-protein cohesin complex. Introduction As the bipolar mitotic spindle forms, sister chromatid pairs become Ubiquitin-mediated proteolysis, discovered over 25 years ago captured by microtubules such that each spindle pole is attached to (Ciechanover et al., 1980; Hershko et al., 1980), covalently attaches only one sister. -

APC/C-Cdh1-Dependent Anaphase and Telophase Progression During
Toda et al. Cell Division 2012, 7:4 http://www.celldiv.com/content/7/1/4 RESEARCH Open Access APC/C-Cdh1-dependent anaphase and telophase progression during mitotic slippage Kazuhiro Toda1, Kayoko Naito1, Satoru Mase1, Masaru Ueno1,2, Masahiro Uritani1, Ayumu Yamamoto1 and Takashi Ushimaru1* Abstract Background: The spindle assembly checkpoint (SAC) inhibits anaphase progression in the presence of insufficient kinetochore-microtubule attachments, but cells can eventually override mitotic arrest by a process known as mitotic slippage or adaptation. This is a problem for cancer chemotherapy using microtubule poisons. Results: Here we describe mitotic slippage in yeast bub2Δ mutant cells that are defective in the repression of precocious telophase onset (mitotic exit). Precocious activation of anaphase promoting complex/cyclosome (APC/ C)-Cdh1 caused mitotic slippage in the presence of nocodazole, while the SAC was still active. APC/C-Cdh1, but not APC/C-Cdc20, triggered anaphase progression (securin degradation, separase-mediated cohesin cleavage, sister- chromatid separation and chromosome missegregation), in addition to telophase onset (mitotic exit), during mitotic slippage. This demonstrates that an inhibitory system not only of APC/C-Cdc20 but also of APC/C-Cdh1 is critical for accurate chromosome segregation in the presence of insufficient kinetochore-microtubule attachments. Conclusions: The sequential activation of APC/C-Cdc20 to APC/C-Cdh1 during mitosis is central to accurate mitosis. Precocious activation of APC/C-Cdh1 in metaphase (pre-anaphase) causes mitotic slippage in SAC-activated cells. For the prevention of mitotic slippage, concomitant inhibition of APC/C-Cdh1 may be effective for tumor therapy with mitotic spindle poisons in humans. -

Functional Interaction Between Bubr1 and Securin in an Anaphase- Promoting Complex/Cyclosomecdc20–Independent Manner
Research Article Functional Interaction between BubR1 and Securin in an Anaphase- Promoting Complex/CyclosomeCdc20–Independent Manner Hyun-Soo Kim,1 Yoon-Kyung Jeon,2 Geun-Hyoung Ha,1 Hye-Young Park,1 Yu-Jin Kim,1 Hyun-Jin Shin,1 Chang Geun Lee,1 Doo-Hyun Chung,2 and Chang-Woo Lee1 1Department of Molecular Cell Biology, Center for Molecular Medicine, Samsung Biomedical Research Institute, Sungkyunkwan University School of Medicine, Suwon, Gyeonggi, Korea and 2Department of Pathology, Seoul National University College of Medicine, Seoul, Korea Abstract Cdc20 (APC/CCdc20). However, Cdc20 in MCC is unable to acti- vate APC/C, as shown by several experiments in which the addi- Activation of the mitotic checkpoint requires the precise tion of Mad2 and/or BubR1 leads to the checkpoint-mediated timing and spatial organization of mitotic regulatory inhibition of APC/CCdc20 activity (2, 10, 11). Silencing of this events, and ensures accurate chromosome segregation. checkpoint signal is initiated by the release of the inhibitory Mitotic checkpoint proteins such as BubR1 and Mad2 bind mitotic checkpoint protein complex from APC/CCdc20; APC/CCdc20 to Cdc20, and inhibit anaphase-promoting complex/cyclo- can drive cells into anaphase by inducing the degradation of someCdc20–mediated securin degradation and the onset of securin (pituitary tumor-transforming gene, PTTG), a small protein anaphase. BubR1 mediates the proper attachment of micro- that inhibits the protease separase and mitotic cyclins (12–15). tubules to kinetochores, and links the regulation of chromo- In metaphase-to-anaphase transition, APC/CCdc20 initiates the some-spindle attachment to mitotic checkpoint signaling. process of chromosome segregation through ubiquitination of Therefore, disruption of BubR1 activity results in a loss securin. -

Intertwined Functions of Separase and Caspase in Cell Division
bioRxiv preprint doi: https://doi.org/10.1101/653584; this version posted May 30, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. Intertwined Functions of Separase and Caspase in Cell Division and Programmed Cell Death Pan-Young Jeong1, Ashish Kumar1, Pradeep Joshi1, and Joel H. Rothman1* Affiliations: 1Department of Molecular, Cellular, and Developmental Biology, and Neuroscience Research Institute, University of California, Santa Barbara, Santa Barbara, CA 93106, USA. *Correspondence to: [email protected] Key words : C. elegans, chromosome separation, separase, caspase, programmed cell death 2 bioRxiv preprint doi: https://doi.org/10.1101/653584; this version posted May 30, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. Abstract: Timely sister chromatid separation, promoted by separase, is essential for faithful chromosome segregation. Separase is a member of the CD clan of cysteine proteases, which also includes the pro-apoptotic enzymes known as caspases. We report that the C. elegans separase SEP-1, primarily known for its role in cell division, is required for apoptosis when the predominant pro-apoptotic caspase CED-3 is compromised. Loss of SEP-1 results in extra surviving cells in a weak ced-3(-) mutant, and suppresses the embryonic lethality of a mutant defective for the apoptotic suppressor ced-9/Bcl-2. We also report apparent non-apoptotic roles for CED-3 in promoting germ cell proliferation and germline meiotic chromosome disjunction and the normal rate of embryonic development. -

A Mutation in Separase Causes Genome Instability and Increased Susceptibility to Epithelial Cancer
Downloaded from genesdev.cshlp.org on September 23, 2021 - Published by Cold Spring Harbor Laboratory Press RESEARCH COMMUNICATION A mutation in separase causes of homozygous cds embryos shows that they also exhibit increased apoptosis as early as 24 hpf, as detected by genome instability and TUNEL staining (Fig. 1B). 5-bromo-2-deoxyuridine increased susceptibility to (BrdU) incorporation also indicates that at 28 hpf, cds embryos have markedly decreased embryonic prolifera- epithelial cancer tion (Supplementary Fig. S1). To further characterize the larger foci phenotype and cell cycle defects exhibited by 1,3 1,4 Jennifer L. Shepard, James F. Amatruda, cds mutant embryos, DNA content analysis was per- David Finkelstein,1 James Ziai,1 K. Rose Finley,1 formed on disaggregated 24-hpf embryos. This study Howard M. Stern,1,2 Ken Chiang,1 demonstrated that cds embryos have a population of Candace Hersey,1 Bruce Barut,1 cells with 8N DNA content, indicating the presence of 2 2 polyploid cells (Fig. 1C). Further analysis also demon- Jennifer L. Freeman, Charles Lee, strated the presence of 16N cells (data not shown). These 2 2 Jonathan N. Glickman, Jeffery L. Kutok, data suggest that cds mutants have a fundamental prob- Jon C. Aster,2 and Leonard I. Zon1,5 lem with control of the mitotic checkpoint. To discover the gene responsible for the cds pheno- 1Children’s Hospital, Boston, Massachusetts 02115, USA; 2 type, a positional cloning project was undertaken and Department of Pathology, Brigham and Women’s Hospital, cds was mapped and localized to linkage group 6 in a Boston, Massacusetts 02115, USA 6.5-centimorgan (cM) interval between the microsatel- lite markers z5294 and z265 (Fig. -

Intertwined Functions of Separase and Caspase in Cell Division and Programmed Cell Death Pan-Young Jeong, Ashish Kumar, Pradeep M
www.nature.com/scientificreports OPEN Intertwined Functions of Separase and Caspase in Cell Division and Programmed Cell Death Pan-Young Jeong, Ashish Kumar, Pradeep M. Joshi & Joel H. Rothman* Timely sister chromatid separation, promoted by separase, is essential for faithful chromosome segregation. Separase is a member of the CD clan of cysteine proteases, which also includes the pro- apoptotic enzymes known as caspases. We report a role for the C. elegans separase SEP-1, primarily known for its essential activity in cell division and cortical granule exocytosis, in developmentally programmed cell death when the predominant pro-apoptotic caspase CED-3 is compromised. Loss of SEP-1 results in extra surviving cells in a weak ced-3(-) mutant, and suppresses the embryonic lethality of a mutant defective for the apoptotic suppressor ced-9/Bcl-2 implicating SEP-1 in execution of apoptosis. We also report apparent non-apoptotic roles for CED-3 in promoting germ cell proliferation, meiotic chromosome disjunction, egg shell formation, and the normal rate of embryonic development. Moreover, loss of the soma-specifc (CSP-3) and germline-specifc (CSP-2) caspase inhibitors result in CED-3-dependent suppression of embryonic lethality and meiotic chromosome non-disjunction respectively, when separase function is compromised. Thus, while caspases and separases have evolved diferent substrate specifcities associated with their specialized functions in apoptosis and cell division respectively, they appear to have retained the residual ability to participate in both processes, supporting the view that co-option of components in cell division may have led to the innovation of programmed cell suicide early in metazoan evolution.