APC/C-Cdh1-Dependent Anaphase and Telophase Progression During
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Polo-Like Kinase 1: Target and Regulator of Anaphase-Promoting Complex/Cyclosome–Dependent Proteolysis Frank Eckerdt1 and Klaus Strebhardt2
Review Polo-Like Kinase 1: Target and Regulator of Anaphase-Promoting Complex/Cyclosome–Dependent Proteolysis Frank Eckerdt1 and Klaus Strebhardt2 1Department of Pharmacology, University of Colorado School of Medicine, Denver, Colorado and 2Department of Gynecology and Obstetrics, Medical School, J.W. Goethe-University, Frankfurt, Germany Abstract to the regulation of the APC/C, an E3 ubiquitin ligase that is Polo-like kinase 1 (Plk1) is a key regulator of progression responsible for the timely destruction of various mitotic proteins, through mitosis. Although Plk1 seems to be dispensable for thereby regulating chromosome segregation, exit from mitosis, entry into mitosis, its role in spindle formation and exit from and a stable subsequent G1 phase (3).The APC/C is first activated by the ancillary protein Cdc20, targeting proteins containing a mitosis is crucial. Recent evidence suggests that a major role Cdc20 of Plk1 in exit from mitosis is the regulation of inhibitors of destruction box (D box), like securin.Once APC/C has the anaphase-promoting complex/cyclosome (APC/C), such as initiated mitotic exit, Cdc20 itself is degraded and is replaced by Cdh1, allowing the degradation of a wider spectrum of substrates. the early mitotic inhibitor 1 (Emi1) and spindle checkpoint Cdh1 proteins. Thus, Plk1 and the APC/C control mitotic regulators The APC/C complex targets not only D box–containing proteins but also proteins exhibiting a KEN box and/or an A box by both phosphorylation and targeted ubiquitylation to Cdh1 ensure the fidelity of chromosome separation at the meta- (e.g., cyclin B1 and Aurora A). Plk1 itself is an APC/C target. -

Lte1 Promotes Mitotic Exit by Controlling the Localization of the Spindle Position Checkpoint Kinase Kin4
Lte1 promotes mitotic exit by controlling the localization of the spindle position checkpoint kinase Kin4 Jill E. Falk1, Leon Y. Chan1,2, and Angelika Amon3 David H. Koch Institute for Integrative Cancer Research and Howard Hughes Medical Institute, Massachusetts Institute of Technology, Cambridge, MA 02139 This contribution is part of the special series of Inaugural Articles by members of the National Academy of Sciences elected in 2010. Contributed by Angelika Amon, May 17, 2011 (sent for review April 27, 2011) For a daughter cell to receive a complete genomic complement, it is The work by Adames et al. (13) proposed a model in which essential that the mitotic spindle be positioned accurately within the interactions between microtubules and the bud neck inhibit the cell. In budding yeast, a signaling system known as the spindle MEN, but how this could lead to Kin4 activation, if indeed Kin4 is position checkpoint (SPOC) monitors spindle position and regulates activated by spindle misposition, is not known. We previously the activity of the mitotic exit network (MEN), a GTPase signaling proposed a model termed the zone model, which posits that the pathway that promotes exit from mitosis. The protein kinase Kin4 budding yeast cell is divided into a MEN inhibitory zone in the is a central component of the spindle position checkpoint. Kin4 mother cell and a MEN activating zone in the daughter cell and primarily localizes to the mother cell and associates with spindle that a sensor, the GTPase Tem1, moves between them. Tem1 as well as most other components of the MEN reside at pole bodies (SPBs) located in the mother cell to inhibit MEN spindle pole bodies (SPBs; yeast centrosomes). -

Patronus Is the Elusive Plant Securin, Preventing Chromosome Separation By
bioRxiv preprint doi: https://doi.org/10.1101/606285; this version posted April 11, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. 1 Patronus is the elusive plant securin, preventing chromosome separation by 2 antagonizing separase 3 Laurence Cromer1, Sylvie Jolivet1, Dipesh Kumar Singh1, Floriane Berthier1, Nancy 4 De Winne2, Geert De Jaeger2, Shinichiro Komaki3,4, Maria Ada Prusicki3, Arp 5 Schnittger3, Raphael Guérois5 and Raphael Mercier1* 6 7 1 Institut Jean-Pierre Bourgin, UMR1318 INRA-AgroParisTech, Université Paris- 8 Saclay, RD10, 78000 Versailles, France. 9 2 Department of Plant Biotechnology and Bioinformatics, Ghent University, Ghent, 10 Belgium, VIB Center for Plant Systems Biology, Ghent, Belgium 11 3 Department of Developmental Biology, Institute for Plant Sciences and 12 Microbiology, University of Hamburg, 22609 Hamburg, Germany. 13 4 Present address : Nara Institute of Science and Technology, Graduate School of 14 Biological Sciences, 8916-5 Takayama, Ikoma, Nara 630-0192, Japan 15 5 Institute for Integrative Biology of the Cell (I2BC), Commissariat à l’Energie 16 Atomique et aux Energies Alternatives (CEA), Centre National de la Recherche 17 Scientifique (CNRS), Université Paris-Sud, CEA-Saclay, Gif-sur-Yvette, France 18 * Corresponding author. [email protected] 19 1 bioRxiv preprint doi: https://doi.org/10.1101/606285; this version posted April 11, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. -

System-Level Feedbacks Control Cell Cycle Progression
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Elsevier - Publisher Connector FEBS Letters 583 (2009) 3992–3998 journal homepage: www.FEBSLetters.org Review System-level feedbacks control cell cycle progression Orsolya Kapuy a, Enuo He a, Sandra López-Avilés b, Frank Uhlmann b, John J. Tyson c, Béla Novák a,* a Oxford Centre for Integrative Systems Biology, Department of Biochemistry, University of Oxford, South Parks Road, Oxford OX1 3QU, UK b Chromosome Segregation Laboratory, Cancer Research UK London Research Institute, 44 Lincoln’s Inn Fields, London WC2A 3PX, UK c Department of Biological Sciences, Virginia Polytechnic Institute and State University, Blacksburg, VA 24061, USA article info abstract Article history: Repetitive cell cycles, which are essential to the perpetuation of life, are orchestrated by an under- Received 25 June 2009 lying biochemical reaction network centered around cyclin-dependent protein kinases (Cdks) and Revised 27 July 2009 their regulatory subunits (cyclins). Oscillations of Cdk1/CycB activity between low and high levels Accepted 13 August 2009 during the cycle trigger DNA replication and mitosis in the correct order. Based on computational Available online 22 August 2009 modeling, we proposed that the low and the high kinase activity states are alternative stable steady Edited by Johan Elf states of a bistable Cdk-control system. Bistability is a consequence of system-level feedback (posi- tive and double-negative feedback signals) in the underlying control system. We have also argued that bistability underlies irreversible transitions between low and high Cdk activity states and Keywords: Cell cycle thereby ensures directionality of cell cycle progression. -

Regulation of Cyclin-Dependent Kinase Activity During Mitotic Exit and Maintenance of Genome Stability by P21, P27, and P107
Regulation of cyclin-dependent kinase activity during mitotic exit and maintenance of genome stability by p21, p27, and p107 Taku Chibazakura*†, Seth G. McGrew‡§, Jonathan A. Cooper§, Hirofumi Yoshikawa*, and James M. Roberts‡§ *Deparment of Bioscience, Tokyo University of Agriculture, 1-1-1 Sakuragaoka, Setagaya-ku, Tokyo 156-8502, Japan; and ‡Howard Hughes Medical Institute and §Division of Basic Sciences, Fred Hutchinson Cancer Research Center, Seattle, WA 98019 Communicated by Robert N. Eisenman, Fred Hutchinson Cancer Research Center, Seattle, WA, February 4, 2004 (received for review October 28, 2003) To study the regulation of cyclin-dependent kinase (CDK) activity bind to and inactivate mitotic cyclin–CDK complexes (15, 16). during mitotic exit in mammalian cells, we constructed murine cell These CKIs accumulate and persist during mid-M-to-G1 phase ͞ lines that constitutively express a stabilized mutant of cyclin A until they are phosphorylated by Sic1 Rum1-resistant G1 cyclin- (cyclin A47). Even though cyclin A47 was expressed throughout CDKs, which initiates their ubiquitin-dependent degradation at mitosis and in G1 cells, its associated CDK activity was inactivated the G1-to-S phase transition (17–19). Thus, Sic1 and Rum1 after the transition from metaphase to anaphase. Cyclin A47 constitute a switch that controls the transition from a state of low associated with both p21 and p27 during mitotic exit, implicating CDK activity to that of high CDK activity, thereby regulating these proteins in CDK inactivation. However, cyclin A47 was fully mitotic exit and S phase entry. This parallels the activity of ؊/؊ ؊/؊ inhibited during the M-to-G1 transition in p21 p27 fibro- APC-Cdh1, and indeed these two pathways constitute redundant blasts. -
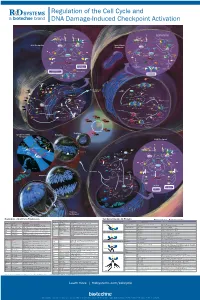
Regulation of the Cell Cycle and DNA Damage-Induced Checkpoint Activation
RnDSy-lu-2945 Regulation of the Cell Cycle and DNA Damage-Induced Checkpoint Activation IR UV IR Stalled Replication Forks/ BRCA1 Rad50 Long Stretches of ss-DNA Rad50 Mre11 BRCA1 Nbs1 Rad9-Rad1-Hus1 Mre11 RPA MDC1 γ-H2AX DNA Pol α/Primase RFC2-5 MDC1 Nbs1 53BP1 MCM2-7 53BP1 γ-H2AX Rad17 Claspin MCM10 Rad9-Rad1-Hus1 TopBP1 CDC45 G1/S Checkpoint Intra-S-Phase RFC2-5 ATM ATR TopBP1 Rad17 ATRIP ATM Checkpoint Claspin Chk2 Chk1 Chk2 Chk1 ATR Rad50 ATRIP Mre11 FANCD2 Ubiquitin MDM2 MDM2 Nbs1 CDC25A Rad50 Mre11 BRCA1 Ub-mediated Phosphatase p53 CDC25A Ubiquitin p53 FANCD2 Phosphatase Degradation Nbs1 p53 p53 CDK2 p21 p21 BRCA1 Ub-mediated SMC1 Degradation Cyclin E/A SMC1 CDK2 Slow S Phase CDC45 Progression p21 DNA Pol α/Primase Slow S Phase p21 Cyclin E Progression Maintenance of Inhibition of New CDC6 CDT1 CDC45 G1/S Arrest Origin Firing ORC MCM2-7 MCM2-7 Recovery of Stalled Replication Forks Inhibition of MCM10 MCM10 Replication Origin Firing DNA Pol α/Primase ORI CDC6 CDT1 MCM2-7 ORC S Phase Delay MCM2-7 MCM10 MCM10 ORI Geminin EGF EGF R GAB-1 CDC6 CDT1 ORC MCM2-7 PI 3-Kinase p70 S6K MCM2-7 S6 Protein Translation Pre-RC (G1) GAB-2 MCM10 GSK-3 TSC1/2 MCM10 ORI PIP2 TOR Promotes Replication CAK EGF Origin Firing Origin PIP3 Activation CDK2 EGF R Akt CDC25A PDK-1 Phosphatase Cyclin E/A SHIP CIP/KIP (p21, p27, p57) (Active) PLCγ PP2A (Active) PTEN CDC45 PIP2 CAK Unwinding RPA CDC7 CDK2 IP3 DAG (Active) Positive DBF4 α Feedback CDC25A DNA Pol /Primase Cyclin E Loop Phosphatase PKC ORC RAS CDK4/6 CDK2 (Active) Cyclin E MCM10 CDC45 RPA IP Receptor -

Control of the Cell Cycle During Meiotic Maturation of Mouse Oocytes
Control of the cell cycle during meiotic maturation of mouse oocytes Ibtissem Nabti Department of Cell and Developmental Biology University College London London, UK A thesis submitted for the degree of Doctor of Philosophy April 2010 Declaration The work presented in this thesis was conducted under the supervision of Professor John Carroll in the department of cell and developmental biology, University College London, and Professor Keith Jones in the department of reproductive physiology, University of Newcastle upon Tyne. I, Ibtissem Nabti confirm that the data presented in this thesis is my own and from original studies. Also, this dissertation has not previously been submitted, wholly or in part, to any other university for any degree or diploma. Ibtissem Nabti 1 Abstract The overall aim of the work presented in this thesis is to better understand the molecular mechanisms underlying the progression of the meiotic division in mammalian oocytes. The first goal of this project was to investigate the relative contribution of securin and cyclin B1 to the control of the protease separase during the indeterminate period of metaphase II arrest. I found here that although there are conditions in which either securin or MPF can prevent chromosome disjunction, such as during meiosis I, securin is the predominant inhibitor of separase during metaphase II arrest. Thus, CDK1 pharmacological inhibition as well as antibody inhibition of CDK1/cyclin B1 binding to separase, both failed to induce sister chromatid disjunction. Instead, securin morpholino knockdown induced sister chromatid separation, which could be rescued by injection of securin cRNA. I also examined the effect of CDK1 and MAPK activities on securin stability. -

A Mathematical Model of Mitotic Exit in Budding Yeast: the Role of Polo Kinase
A Mathematical Model of Mitotic Exit in Budding Yeast: The Role of Polo Kinase Baris Hancioglu*¤, John J. Tyson Department of Biological Sciences, Virginia Polytechnic Institute and State University, Blacksburg, Virginia, United States of America Abstract Cell cycle progression in eukaryotes is regulated by periodic activation and inactivation of a family of cyclin–dependent kinases (Cdk’s). Entry into mitosis requires phosphorylation of many proteins targeted by mitotic Cdk, and exit from mitosis requires proteolysis of mitotic cyclins and dephosphorylation of their targeted proteins. Mitotic exit in budding yeast is known to involve the interplay of mitotic kinases (Cdk and Polo kinases) and phosphatases (Cdc55/PP2A and Cdc14), as well as the action of the anaphase promoting complex (APC) in degrading specific proteins in anaphase and telophase. To understand the intricacies of this mechanism, we propose a mathematical model for the molecular events during mitotic exit in budding yeast. The model captures the dynamics of this network in wild-type yeast cells and 110 mutant strains. The model clarifies the roles of Polo-like kinase (Cdc5) in the Cdc14 early anaphase release pathway and in the G-protein regulated mitotic exit network. Citation: Hancioglu B, Tyson JJ (2012) A Mathematical Model of Mitotic Exit in Budding Yeast: The Role of Polo Kinase. PLoS ONE 7(2): e30810. doi:10.1371/ journal.pone.0030810 Editor: Michael Lichten, National Cancer Institute, United States of America Received October 8, 2011; Accepted December 21, 2011; Published February 23, 2012 Copyright: ß 2012 Hancioglu, Tyson. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. -

A Novel Function for HSF1-Induced Mitotic Exit Failure and Genomic Instability Through Direct Interaction Between HSF1 and Cdc20
Oncogene (2008) 27, 2999–3009 & 2008 Nature Publishing Group All rights reserved 0950-9232/08 $30.00 www.nature.com/onc ORIGINAL ARTICLE A novel function for HSF1-induced mitotic exit failure and genomic instability through direct interaction between HSF1 and Cdc20 YJ Lee1, HJ Lee1, JS Lee2, D Jeoung3, CM Kang1, S Bae1, SJ Lee2, SH Kwon4, D Kang4 and YS Lee1 1Division of Radiation Effect, Korea Institute of Radiological and Medical Sciences, Seoul, Republic of Korea; 2Division of Radiation Cancer Research, Korea Institute of Radiological and Medical Sciences, Seoul, Korea; 3College of Natural Sciences, Kangwon National University, Chunchon, Korea and 4Korea Basic Science Institute, Chunchon Center, Chunchon, Korea Although heat-shock factor (HSF) 1 is a known highly aneuploid, however, the molecular mechanisms transcriptional factor of heat-shock proteins, other path- underlying the development of aneuploidy have not wayslike production of aneuploidy and increasedprotein been fully defined, although mutations in mitotic stability of cyclin B1 have been proposed. In the present checkpoint genes have been identified in a subset of study, the regulatory domain of HSF1 (amino-acid human cancers and cell lines (Lengauer et al., 1997; sequence 212–380) was found to interact directly with Cahill et al., 1998). the amino-acid sequence 106–171 of Cdc20. The associa- Enhanced heat-shock protein (HSP) expression in tion between HSF1 and Cdc20 inhibited the interaction response to various stimuli is regulated by heat-shock between Cdc27 and Cdc20, the phosphorylation of Cdc27 factors (HSFs), the functional relevance of which is now and the ubiquitination activity of anaphase-promoting emerging. -

A Dynamical Model of Mitotic Exit in Budding Yeast
A Dynamical Model of Mitotic Exit in Budding Yeast Baris Hancioglu1, Kathy Chen2, and John J. Tyson3 any cell cycle stage [3] [4]. Cdc5 is not only part of MEN, Short Abstract — We have developed a nonlinear ordinary but also part of FEAR, and can induce Cdc14 release even differential equations model for the control of Cdc14, an when other FEAR and MEN components are silent (ii) Net1 essential phosphatase promoting mitotic exit in yeast. The has multiple phosphorylation sites. The model incorporates model captures the dynamics of mitotic exit in wild-type and multi-phosphorylation of Net1 by protein kinases; Cdk, dozens of mutant cells clarifying the roles of Esp1 and Cdc5 Cdc5, and the Dbf2/Mob1 kinase in the MEN pathway[5] (Polo kinase) in mitotic exit pathways. Understanding how [6] (iii) Cdc15 acts downstream of Tem1 in MEN network. Polo-like kinase fits into the exit pathway is important because it is being actively pursued as a therapeutic target in the Even Tem1 is inactive, overexpressed CDC15 can still make treatment of human cancer. MEN active and sustain Cdc14 release. (iv) Net1 phosphorylation at Cdk consensus sites is an important part Keywords — Cell Cycle, Exit from Mitosis, Polo kinase of FEAR, however it is not an essential requirement for (Cdc5), Cdc14, Separase (Esp1), Mitotic Exit Network (MEN). mitotic exit events [7]. I. BACKGROUND HE Cell cycle events in eukaryotes are regulated by III. CONCLUSION Tperiodic activation and inactivation of a family of We propose a novel mechanism for multiphosphorylation of cyclin–dependent kinases (Cdks). Entry into mitosis is Net1 by several kinases: Cdk, Cdc5 (Polo) and Dbf2/Mob1 initiated by accumulation of Cdk in complexes with B-type (through activation of Cdc15). -

Functional Interaction Between Bubr1 and Securin in an Anaphase- Promoting Complex/Cyclosomecdc20–Independent Manner
Research Article Functional Interaction between BubR1 and Securin in an Anaphase- Promoting Complex/CyclosomeCdc20–Independent Manner Hyun-Soo Kim,1 Yoon-Kyung Jeon,2 Geun-Hyoung Ha,1 Hye-Young Park,1 Yu-Jin Kim,1 Hyun-Jin Shin,1 Chang Geun Lee,1 Doo-Hyun Chung,2 and Chang-Woo Lee1 1Department of Molecular Cell Biology, Center for Molecular Medicine, Samsung Biomedical Research Institute, Sungkyunkwan University School of Medicine, Suwon, Gyeonggi, Korea and 2Department of Pathology, Seoul National University College of Medicine, Seoul, Korea Abstract Cdc20 (APC/CCdc20). However, Cdc20 in MCC is unable to acti- vate APC/C, as shown by several experiments in which the addi- Activation of the mitotic checkpoint requires the precise tion of Mad2 and/or BubR1 leads to the checkpoint-mediated timing and spatial organization of mitotic regulatory inhibition of APC/CCdc20 activity (2, 10, 11). Silencing of this events, and ensures accurate chromosome segregation. checkpoint signal is initiated by the release of the inhibitory Mitotic checkpoint proteins such as BubR1 and Mad2 bind mitotic checkpoint protein complex from APC/CCdc20; APC/CCdc20 to Cdc20, and inhibit anaphase-promoting complex/cyclo- can drive cells into anaphase by inducing the degradation of someCdc20–mediated securin degradation and the onset of securin (pituitary tumor-transforming gene, PTTG), a small protein anaphase. BubR1 mediates the proper attachment of micro- that inhibits the protease separase and mitotic cyclins (12–15). tubules to kinetochores, and links the regulation of chromo- In metaphase-to-anaphase transition, APC/CCdc20 initiates the some-spindle attachment to mitotic checkpoint signaling. process of chromosome segregation through ubiquitination of Therefore, disruption of BubR1 activity results in a loss securin. -

Toward a Systems-Level View of Mitotic Checkpoints
Toward a systems-level view of mitotic checkpoints Bashar Ibrahim Bio System Analysis Group, Friedrich-Schiller-University Jena, and Jena Centre for Bioinformatics (JCB), 07743 Jena, Germany. E-Mail: [email protected]; Tel.: +49-3641-9-46460; Fax +49-3641-9-46302. Summary Reproduction and natural selection are the key elements of life. In order to reproduce, the genetic material must be doubled, separated and placed into two new daughter cells, each containing a complete set of chromosomes and organelles. In mitosis, transition from one process to the next is guided by intricate surveillance mechanisms, known as the mitotic checkpoints. Dis-regulation of cell division through checkpoint malfunction can lead to developmental defects and contribute to the development or progression of tumors. This review approaches two important mitotic checkpoints, the spindle assembly checkpoint (SAC) and the spindle position checkpoint (SPOC). The highly conserved spindle assembly checkpoint (SAC) controls the onset of anaphase by preventing premature segregation of the sister chromatids of the duplicated genome, to the spindle poles. In contrast, the spindle position checkpoint (SPOC), in the budding yeast S. cerevisiae, ensures that during asymmetric cell division mitotic exit does not occur until the spindle is properly aligned with the cell polarity axis. Although there are no known homologs, there is indication that functionally similar checkpoints exist also in animal cells. Keywords: Systems biology; kinetochore; spindle assembly checkpoint; spindle position checkpoint 1 1. Introduction Correct DNA segregation during mitosis is a fundamental process that ensures the faithful inheritance of genomic information for the propagation of cell life. Segregation ( Figure 1 ) failures underlie many human health problems, most notably aneuploidy and cancer (Holland and Cleveland, 2009; Suijkerbuijk and Kops, 2008).