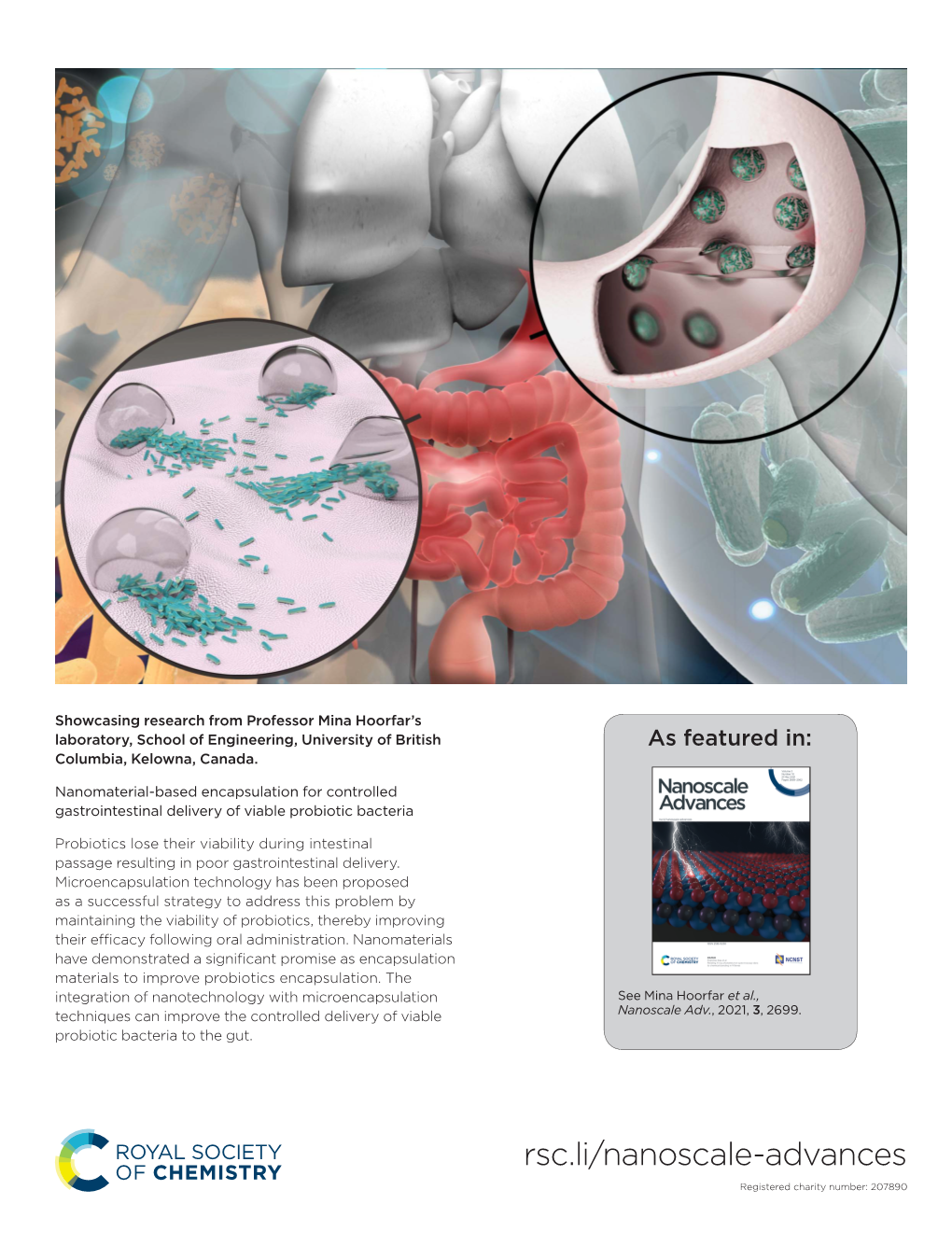
Nanomaterial-Based Encapsulation for Controlled Gastrointestinal Delivery of Viable Probiotic Bacteria
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Cell Encapsulation: Promise and Progress
COMMENTARY In cell encapsulation, transplanted cells are protected from immune rejection by an artificial, semipermeable membrane, potentially allowing transplantation (allo- or xenotransplantation) without the need for immunosuppression. Yet, despite some promising results in animal studies, the field has not lived up to expectations, and clinical products based on encapsulated cell technology continue to elude the scientific community. This commentary discusses the reasons for this, summarizes recent progress in the field and outlines what is needed to bring this technology closer to clinical application. Cell encapsulation: Promise and progress In 1964, T.M.S. Chang1 proposed the idea Some of the important considerations of using ultrathin polymer membrane GORKA ORIVE1, for consistent clinical success of cell en- microcapsules for the immunoprotection ROSA MARÍA HERNÁNDEZ1, capsulation include a source of func- of transplanted cells and introduced the ALICIA R. GASCÓN1, tional cells; a biocompatible, as well as term ‘artificial cells’ to define the concept RICCARDO CALAFIORE2, mechanically and chemically stable, of bioencapsulation, which was success- THOMAS M.S. CHANG3, membrane of a suitable permeability fully implemented 20 years later to im- PAUL DE VOS4, cut-off value that provides immune pro- mobilize xenograft islet cells. When GONZALO HORTELANO5, tection to the implant; functional per- implanted into rats, the microencapsu- DAVID HUNKELER6, IGOR LACÍK7, formance; biosafety; and long-term lated islets corrected the diabetic state for A.M. JAMES SHAPIRO8 & survival of the graft. several weeks2. Since then, there has been JOSÉ LUIS PEDRAZ1 considerable progress toward understand- Lack of clinical-grade polymers. In http://www.nature.com/naturemedicine ing the biological and technological requirements for successful the quest for a better microencapsulation design, many transplantation of encapsulated cells in experimental animal types of natural and synthetic polymers are being explored. -

ALS) with Mutations in Angiogenin and Superoxide Dismutase 1
Therapeutic strategies for the treatment of Amyotrophic Lateral Sclerosis (ALS) with mutations in Angiogenin and Superoxide Dismutase 1 by Krishna Chaitanya Aluri Bachelor of Pharmacy, Rajiv Gandhi University of Health Sciences Master of Science in Biopharmaceutical Science, Northeastern University A dissertation submitted to The Faculty of the College of Science of Northeastern University in partial fulfillment of the requirements for the degree of Doctor of Philosophy April, 16, 2020 Dissertation directed by Jeffrey N. Agar Professor of Chemistry and Chemical Biology 1 Dedication “When I walk, I walk with you. Where I go, you're with me always.” ― Alice Hoffman, The Story Sisters, 2009. I dedicate this work to my family and friends. A special thanks to my parents Aluri Gopal Rao and Mallela Visalakshi; brother Venkata Vishnuvardan Aluri and wife Prathyusha Gundlapally for their inspiration and words of encouragement. I also dedicate this work to my friends Husain Attarwala, Arnik Shah, Aatman Doshi, Kirtika Asrani, Smith Patel and Ranjitha Gaddipati for their support. 2 Acknowledgments I would like to express my deep and sincere gratitude to Prof. Jeffrey N. Agar for continuous support and guidance. I would like to thank my fellow labmates Dr. Joseph P. Salisbury, Dr. Daniel P. Donnelly, Md. Amin Hossain, Durgalakshmi Sivasankar, and Nicholas D. Schmitt for their contributions and thoughtful discussions. I thank my thesis committee Prof. Alexander Ivanov, Prof. Ke Zhang, Dr. Jared R. Auclair, Dr. Roman Manetsch, and Dr. Saeho Chong for their insightful comments, time, and encouragement. I would like to thank our collaborators Dr. Jochen H.M. Prehn, Dr. Roman Manetsch, Dr. -

3:15 P.M. 230. Bio-Inspired Design of a Retrievable and Scalable Cell
Bio-inspired design of a retrievable and scalable cell encapsulation device for potential treatment of type 1 diabetes Minglin Ma Biological and Environmental Engineering, Cornell University Statement of Purpose: Cell encapsulation has been glucagon in the islets retrieved from both kidney capsule shown to hold promise for effective, long-term treatment and TRAFFIC, similar to the islets before transplantation. Human islets - kidney capsule (n=9) 500 Human islets - TRAFFIC (n=10) c Insulin Nkx6.1 of type 1 diabetes (T1D) (1-4), an unrelenting disease that a Non-diabetic control (n=4) Glucagon 450 Diabetic control (n=21) *# Nucleus 400 Transplantation Before affects millions of people. However, encapsulating 350 transplantation 300 systems developed to date still face various challenges. 250 100 µm 100 µm 200 150 Insulin Nkx6.1 For example, alginate hydrogel capsules, despite their 100 Glucagon Nucleus 0 20 40 60 80 100 120 140 160 180 Retrieved from biocompatibility and function, are difficult to retrieve or (mg/dL) glucose concentration Blood Time after STZ injections (day) Human islets - kidney capsule (n=9) Kidney capsule b Human islets - TRAFFIC (n=10) Non-diabetic control (n=4) 30 #* Diabetic control (n=21) replace in completeness due to the large number of 100 µm 100 µm 28 Transplantation 26 Insulin Nkx6.1 capsules required for effective treatment and the Glucagon 24 Nucleus complicated organ structures in the transplantation site 22 Retrieved from TRAFFIC Body weight weight (g) Body 20 18 (i.e. peritoneal space), contributing to risks and concerns 100 µm 100 µm 0 20 40 60 80 100 120 140 160 180 in case of transplant failure or medical complications. -

Cell Encapsulation: Overcoming Barriers in Cell Transplantation in Diabetes and Beyond
Advanced Drug Delivery Reviews 139 (2019) 92–115 Contents lists available at ScienceDirect Advanced Drug Delivery Reviews journal homepage: www.elsevier.com/locate/addr Cell encapsulation: Overcoming barriers in cell transplantation in diabetes and beyond Marco Farina a,b,1, Jenolyn F. Alexander a,1, Usha Thekkedath a, Mauro Ferrari a, Alessandro Grattoni a,⁎ a Department of Nanomedicine, Houston Methodist Research Institute, Houston, TX, United States b Department of Electronics and Telecommunications, Politecnico di Torino, Torino, Italy article info abstract Article history: Cell-based therapy is emerging as a promising strategy for treating a wide range of human diseases, such as di- Received 30 January 2018 abetes, blood disorders, acute liver failure, spinal cord injury, and several types of cancer. Pancreatic islets, Received in revised form 19 March 2018 blood cells, hepatocytes, and stem cells are among the many cell types currently used for this strategy. The encap- Accepted 25 April 2018 sulation of these “therapeutic” cells is under intense investigation to not only prevent immune rejection but also Available online 30 April 2018 provide a controlled and supportive environment so they can function effectively. Some of the advanced encap- Keywords: sulation systems provide active agents to the cells and enable a complete retrieval of the graft in the case of an Cell-based therapy adverse body reaction. Here, we review various encapsulation strategies developed in academic and industrial Encapsulation system settings, including the state-of-the-art technologies in advanced preclinical phases as well as those undergoing Stem cells clinical trials, and assess their advantages and challenges. We also emphasize the importance of stimulus- 3D printing responsive encapsulated cell systems that provide a “smart and live” therapeutic delivery to overcome barriers Pancreatic islets in cell transplantation as well as their use in patients. -

Porcine Islet Encapsulation for Treatmet of Type-1 Diabetes Using Precision Particle Fabrication Method
PORCINE ISLET ENCAPSULATION FOR TREATMET OF TYPE-1 DIABETES USING PRECISION PARTICLE FABRICATION METHOD BY BENJAMIN LEW THESIS Submitted in partial fulfillment of the requirements for the degree of Master of Science in Bioengineering in the Graduate College of the University of Illinois at Urbana-Champaign, 2016 Urbana, Illinois Advisers: Professor Kyekyoon (Kevin) Kim Research Professor Hyungsoo Choi ABSTRACT Type-1 diabetes (T1D), also known as insulin-dependent diabetes, is characterized by the inability of pancreas to produce sufficient amount of insulin to regulate blood glucose level in the body. Long-term complications such as nephropathy, neuropathy, retinopathy and cardiovascular disease make T1D a major health problem throughout the world. For the past few decades, allotransplantation of insulin-producing islets from the pancreas of deceased human donors has shown promising results in the restoration and sustenance of normoglycemia. Islet transplantation is a suitable procedure, especially for adolescents since it has substantially lower risk in operating procedure than whole-pancreas transplantation. However, only small percentage of patients of T1D can be treated with islet allotransplantation because of the limited donor availability. Xenotransplantation using porcine islet is a good alternative treatment of T1D. Porcine islet is an ideal substitute of beta cell function for human islet for several reasons: availability, compatibility and functional similarity to human islets. In recent years, treatment with porcine islets has shown significant progress of providing sustained normoglycemia in diabetes-induced nonhuman primates (NHPs), which has demonstrated the feasibility of clinical xenotransplantation. Yet, there are still a number of barriers to overcome in order to accomplish successful clinical application for xenotransplantation. -

Advances and Barriers in Mammalian Cell Encapsulation for Treatment of Diabetes
Immun., Endoc. & Metab. Agents in Med. Chem., 2006, 6, 139-153 139 Advances and Barriers in Mammalian Cell Encapsulation for Treatment of Diabetes Paul de Vos1,*, A. Andersson2, S.K. Tam3, M.M. Faas1 and J.P. Hallé3 1Department of Pathology and Laboratory Medicine, University Hospital of Groningen, Hanzeplein 1, 9700 RB Groningen, The Netherlands; 2Department of Medical Cell Biology, University of Uppsala, PO Box 571, SE-75123 Uppsala, Sweden and 3Centre de recherche Guy-Bernier, Hôpital Maisonneuve-Rosemont, Université de Montréal, Montréal, Québec, Canada, H1T 2M4 Abstract: Mammalian cell encapsulation is under investigation for the treatment of a wide variety of diseases, since it allows for transplantation of endocrine cells in the absence of undesired immunosuppression. The technology is based on the principle that transplanted tissue is protected for the host immune system by an artificial membrane. In spite of the simplicity of the concept, progress in the field of immuno-isolation has been hampered. During the past two decades, three major approaches of encapsulation have been studied. These include (i) intravascular macrocapsules, which are anastomosed to the vascular system as AV shunt, (ii) extravascular macrocapsules, which are mostly diffusion chambers transplanted at different sites, and (iii) extravascular microcapsules transplanted in the peritoneal cavity. The advantages and pitfalls of the three approaches are discussed and compared in view of applicability in clinical islet transplantation. At present, microcapsules, due to their spatial characteristics, offer better diffusion capacity than mac- rocapsules. During the past five years, important advances have been made in the knowledge of the characteristics and requirements capsules have to meet in order to provide optimal biocompatibility and survival of the enveloped tissue. -

Islet Encapsulation: Physiological Possibilities and Limitations
1748 Diabetes Volume 66, July 2017 Islet Encapsulation: Physiological Possibilities and Limitations Olle Korsgren Diabetes 2017;66:1748–1754 | https://doi.org/10.2337/db17-0065 A logical cure for type 1 diabetes (T1D) involves replacing insulin-producing cells will be available for clinical testing. the lost insulin-producing cells with new ones, preferably The particular problems of generating glucose-responsive cells from a well-characterized and unlimited source of insulin-producing cells were recently elegantly reviewed human insulin-producing cells. This straightforward and and will not further be discussed in this Perspective (3). simple solution to provide a cure for T1D is immensely The current indications for clinical islet transplantation attractive but entails at least two inherent and thus far are 1) severe unawareness of hypoglycemia and 2) trans- 1 unresolved hurdles: ) provision of an unlimited source of plantation to subjects already on systemic immunosuppres- 2 functional human insulin-producing cells and )preven- sion, most often because of a previous or simultaneous tion of rejection without the side effects of systemic kidney transplant. At present, there is no major shortage immunosuppression. Generation of transplantable insulin- of primary human islets, i.e., those obtained from organ producing cells from human embryonic stem cells or in- donors, to fulfill the current indications for clinical islet duced pluripotent stem cells is at present close to reality, transplantation; a shortage of insulin-producing cells will and we are currently awaiting the first clinical studies. Focus is now directed to foster development of novel only occur if we can control immune-mediated rejection means to control the immune system to enable large- of the transplanted cells without the need for systemic scale clinical application. -

Embryonic Stem Cells As a Cell Source for Tissue Engineering
CHAPTER 32 Embryonic Stem Cells as a Cell Source for Tissue Engineering Ali Khademhosseini1,2, Jeffrey M. Karp1,2, Sharon Gerecht-Nir3, Lino Ferreira3, Nasim Annabi1,2, Dario Sirabella4, Gordana Vunjak-Novakovic4 and Robert Langer1,3 1 Harvard-Massachusetts Institute of Technology Division of Health Sciences and Technology, Massachusetts Institute of Technology, Cambridge, Massachusetts 2 Department of Medicine, Brigham and Women’s Hospital, Harvard Medical School, Boston, Massachusetts 3 Department of Chemical Engineering, Massachusetts Institute of Technology, Cambridge, Massachusetts 4 Department of Biomedical Engineering and Department of Medicine, Columbia University, New York, New York 609 INTRODUCTION It has been estimated that approximately 3,000 people die every day in the US from dis- eases that could have been treated with stem cell-derived tissues [1]. Given the therapeutic potential and growing public awareness of stem cells to treat disease, it is not surprising that embryonic stem cell (ESC) research has been rapidly expanding since mouse ESCs (mESCs) were first isolated in 1981 [2,3] followed by the isolation of human embryonic stem cells (hESCs) in 1998 [4,5] from the inner cell mass (ICM) of human blastocysts (Fig. 32.1). Adult stem cells have been used clinically since the 1960s for therapies such as bone marrow transplantation, and these cells hold great therapeutic promise. ESCs also offer major benefits, including their ease of isolation, ability to propagate rapidly without differentiation, and e most significantly e their potential to form all cell types in the body. Additionally, ESCs are an attractive cell source for the study of developmental biology, drug/toxin screening studies, and the development of therapeutic agents to aid in tissue or organ replacement therapies. -

Cell Encapsulation Could Enhance Antiviral Vaccines
Rue Gabrielle-Perret-Gentil 4 CH – 1211 Genève 14 Press Release Geneva, 27 May 2021 CELL ENCAPSULATION COULD ENHANCE ANTIVIRAL VACCINES Immunotherapy techniques developed in oncology to combat cancerous cells have great potential for fighting viruses. A research team from the University Hospitals of Geneva (HUG) and the University of Geneva (UNIGE), in Switzerland, in collaboration with MaxiVAX, a spinoff of both institutions, developed an innovative technology called "cell encapsulation". Originally designed to stimulate immunity to fight cancer, the COVID-19 pandemic motivated the scientists to broaden the scope of their technology to test its effectiveness against viruses. The first results of a pre-clinical study are very encouraging and can be discovered in the journal Vaccines. The immune system is able to identify cancer cells and fight them, just as it does against a viral or bacterial pathogen. Researchers therefore rely on this to develop vaccines against cancer. "To develop an effective vaccine, two elements are needed: a target recognized by the immune system, such as cells, proteins, DNA or RNA sequences, and an adjuvant able to effectively stimulate the immune response", says Nicolas Mach, an oncologist at the HUG Division of Oncology, and a professor at UNIGE Faculty of Medicine Department of Medicine and Translational Research Centre in Onco-Haematology, and co-author of this study. To this end, Nicolas Mach and his team, in partnership with the company MaxiVAX, developed a novel cell-based cancer vaccination using cell encapsulation technology. With the coronavirus pandemic and the need to develop effective vaccines, they decided to extend the scope of this technology to test its effectiveness against viruses. -

Encapsulation of Transgenic Cells for Gene Therapy
Chapter 8 Encapsulation of Transgenic Cells for Gene Therapy Wujie Zhang Additional information is available at the end of the chapter http://dx.doi.org/10.5772/61050 Abstract A major challenge to emerging cell-based medicine including gene therapy is the host immune rejection of transplanted donor cells or engineered tissue. One way to address this problem is to use drugs to achieve immunosuppression. However, suppressing the patient’s immune system may put the patient at risk for many other diseases. An alternative is to encapsulate living cells in macro/microcapsules to achieve immunoi‐ solation of the cells, thereby increasing cell viability in the patient’s body following transplantation. The capsule’s membrane protects the encapsulated cells from being damaged by both the host’s immune system and mechanical stress while allowing free diffusion of nutrients and metabolic waste for the cells to survive. Moreover, the membrane could be designed to achieve controlled and/or sustained release of therapeutic products produced by the encapsulated transgenic cells to treat a variety of diseases such as cardiovascular disorders, anemia, wounds, bone fractures, and cancer. Keywords: Cell microencapsulation, Encapsulation, Microcapsules, Gene therapy, Cell-based medicine 1. Introduction Cell encapsulation is the process of entrapping cells into a matrix. In general, the matrix is spherical in shape and in the form of a polymeric hydrogel. Cell encapsulation technology has shown great promise for immunoisolation and controlled release of therapeutic products towards gene therapy. Figure 1 demonstrates the mechanism of encapsulated transgenic cells for gene therapy. © 2015 The Author(s). Licensee InTech. This chapter is distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/3.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. -

Ciliary Neurotrophic Factor Cell-Based Delivery Prevents Synaptic Impairment and Improves Memory in Mouse Models of Alzheimer’S Disease
7516 • The Journal of Neuroscience, June 2, 2010 • 30(22):7516–7527 Behavioral/Systems/Cognitive Ciliary Neurotrophic Factor Cell-Based Delivery Prevents Synaptic Impairment and Improves Memory in Mouse Models of Alzheimer’s Disease Pierre Garcia,1* Ihsen Youssef,1* Jo K. Utvik,2 Sabrina Florent-Be´chard,1 Vanassa Barthe´le´my,2 Catherine Malaplate-Armand,1,4 Badreddine Kriem,1 Christophe Stenger,1 Violette Koziel,1 Jean-Luc Olivier,1,4 Marie-Christine Escanye,1,4 Marine Hanse,1 Ahmad Allouche,1 Ce´dric Desbe`ne,1,4 Frances T. Yen,1 Rolf Bjerkvig,2,3 Thierry Oster,1,5 Simone P. Niclou,2 and Thierry Pillot1 1JE2482 Lipidomix, Nancy Universite´, 54500 Vandoeuvre, France, 2NorLux Neuro-Oncology Laboratory, Centre de Recherche Public-Sante´, L-1526 Luxembourg, 3NorLux Neuro-Oncology, Department of Biomedicine University of Bergen, N-5020 Bergen, Norway, 4Department of Biochemistry, University Hospital, F-54000 Nancy, France, and 5Unite´ de Formation et de Recherche, Sciences Fondamentales et Applique´es, Universite´ Paul Verlaine, 57000 Metz, France The development of novel therapeutic strategies for Alzheimer’s disease (AD) represents one of the biggest unmet medical needs today. Applicationofneurotrophicfactorsabletomodulateneuronalsurvivalandsynapticconnectivityisapromisingtherapeuticapproachfor AD. We aimed to determine whether the loco-regional delivery of ciliary neurotrophic factor (CNTF) could prevent amyloid- (A) oligomer-induced synaptic damages and associated cognitive impairments that typify AD. To ensure long-term administration of CNTF in the brain, we used recombinant cells secreting CNTF encapsulated in alginate polymers. The implantation of these bioreactors in the brain of A oligomer-infused mice led to a continuous secretion of recombinant CNTF and was associated with the robust improvement of cognitive performances. -

Localized Immunosuppression Therapy for Islet Cell Encapsulation Madeline R
The Summer Undergraduate Research Fellowship (SURF) Symposium 3 August 2017 Purdue University, West Lafayette, Indiana, USA Localized Immunosuppression Therapy for Islet Cell Encapsulation Madeline R. McLaughlin, Clarissa Stephens, Sherry Voytik-Harbin Weldon School of Biomedical Engineering, Purdue University ABSTRACT Type 1 diabetes, an autoimmune disease in which the body’s immune system destroys the insulin-producing beta cells necessary for managing a person’s blood glucose levels, affects 1.25 million Americans. A potential treatment for this disease is islet cell transplantation where Islets of Langerhans, containing the beta cells, are transplanted from a normal donor to a diabetic recipient to regulate blood glucose levels and provide insulin independence. Similar to whole organ transplantation, immune modulation through immunosuppression therapy is necessary for successful transplantation of islets without rejection. However, long-term systemic immunosuppression therapy can be toxic to the patient and the islets. Because of this, there is a need for alternative strategies where immune protection is provided locally through islet encapsulation or localized delivery of immunomodulatory agents. In this study, we investigated the toxicity of the inactive and active forms of an FDA approved immunosuppressant drug by assessing the morphology, viability, and protein expression of human islets embedded in a novel collagen encapsulation material. Overall, there was decreased islet cell death and improved morphology with i) decreasing drug concentration from 1.0 mg/mL to 0.01 mg/mL; ii) use of the inactive form of the drug vs the active form of the drug; and iii) incorporation of the drug into the collagen encapsulation material rather than the culture medium.