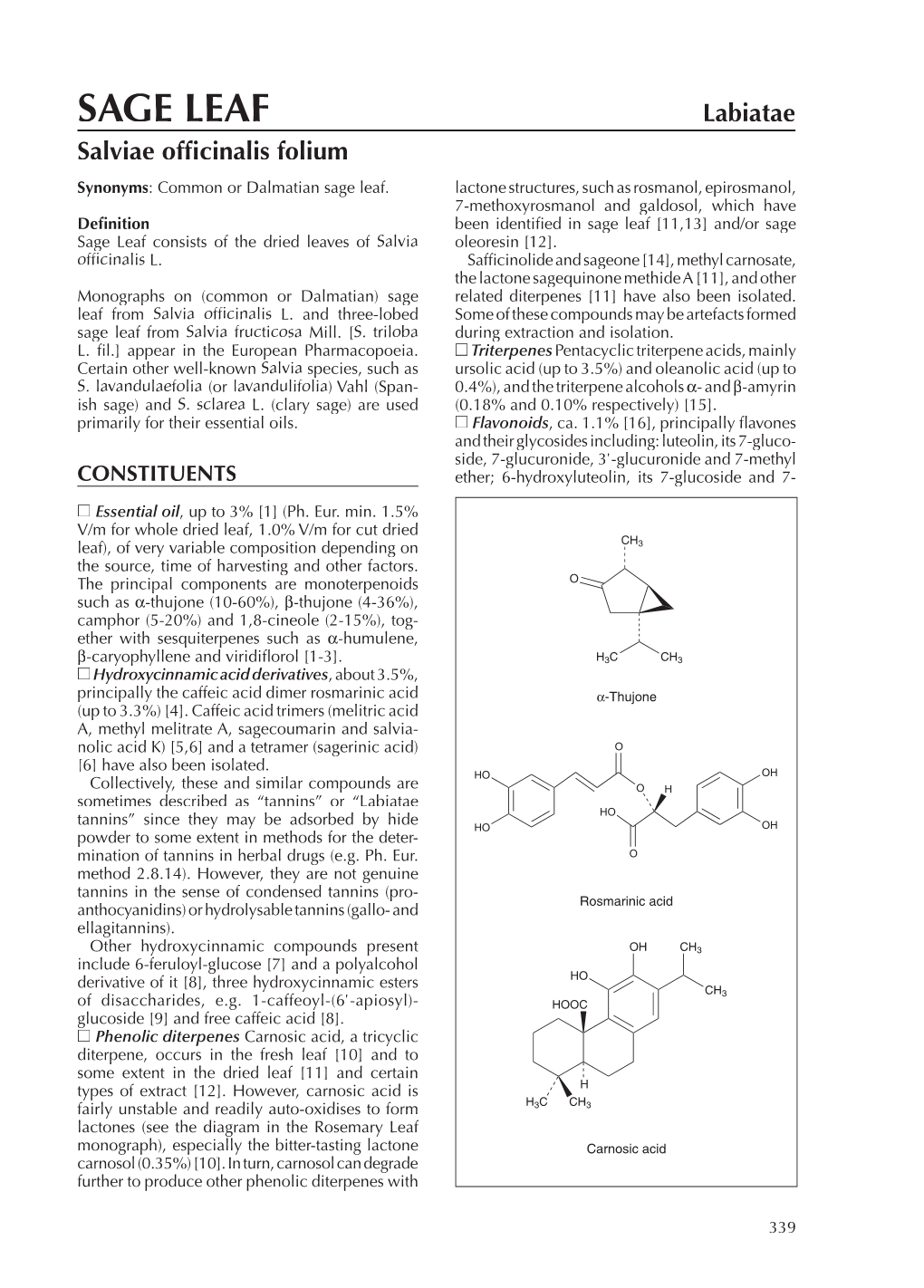
SAGE LEAF Labiatae Salviae Offi Cinalis Folium Synonyms: Common Or Dalmatian Sage Leaf
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Mint in the Garden Kristie Buckland and Dan Drost Vegetable Specialist
Revised May 2020 Mint in the Garden Kristie Buckland and Dan Drost Vegetable Specialist Summary Plants: Mint can be grown from seed or Mint is a rapid growing perennial herb with transplants. Since mints readily hybridize between many varieties that grow up to 3 feet tall and are quite different types, plants grown from seed often fail to be invasive. Mint grows best in full sun to partial shade, true to type. For specific cultivars or varieties, buy should be planted early in the growing season and is established plants from reputable sources, take cuttings generally hardy to -20° F. Mint prefers moist soil from known plants, or divide an established plant. conditions, but excess water will promote root and leaf Divide and replant established plants in the spring diseases. Harvest leaves and stems throughout the before growth starts or early in the fall. season, or cut back within an inch of the ground about Planting and Spacing: Sow seeds ¼ inch deep three times a season, just before the plant blooms. and then thin seedlings once they emerge. Transplants should be planted with roots just beneath the soil Varieties surface. Row spacing should be at least 2 feet apart to allow for growth. Use care when selecting mint varieties. The taste Water: Water regularly during the growing and smell varies greatly between varieties. For cold areas season, supplying up to 1 to 2 inches per week, of Utah, peppermint, spearmint, and woolly mints are depending on temperatures, exposure and soil very hardy. All varieties are well suited to areas of Utah conditions. -

PDF Transcript of This Episode Here
Medicine Stories Podcast Episode 39 with Kami McBride Kitchen Herbalism: The Body Remembers February 14, 2019 [0:00:00] (Excerpt from today’s show by Kami McBride) Kami: And so, my goal is to get your pantry filled with herbal condiments that make every meal a little bit better, and truly, it’s the best health care prevention around is what you’ve got in your kitchen and what you put on your food. You know, an herbal lifestyle is good as a health care plan as anything we can do to support our health. (Intro Music: acoustic guitar folk song "Wild Eyes" by Mariee Sioux) [0:00:26] Amber: Hey friends! Welcome to the Medicine Stories podcast, where we are remembering what it is to be human upon the earth. This is Episode 39, and I am Amber Magnolia Hill. And today we are talking again to Kami McBride, who was my guest on Episode 20, and you all LOVED her just as much as I love her. It’s really exciting having her back. Kami’s book, The Herbal Kitchen, is having its tenth anniversary right now and is being reissued in a brand new format with more recipes, more photos, and so I asked her to come back and talk to me about Kitchen Herbalism. I really love Kami’s focus and what she taught me in my year-long apprenticeship with her in 2007 is on home herbalism. I feel like we have a tendency to really overcomplicate herbalism for people who are new. Just feeling called to learn more about working with plants, it can seem so overwhelming, and where do you even begin, and how are you going to know everything? Which, you’re not, which Kami and I talked about in that first interview. -

Sage Salvia Officinalis Garden Sage, Red Sage, Salvia Salvatrix Photo
Sage Salvia officinalis Garden Sage, Red Sage, Salvia salvatrix Photo: LuvlyMikimoto 9/1/07 Botanical Description Salvia officinalis can be used for both culinary and medical needs. Sage generally grows to be approximately a foot tall with leaves one and a half to two inches long. The leaves grow in pairs on the thin stems. Over time the plant eventually becomes woody and much like a shrubbery. All parts of the plant have a strong scent due to the essential oils within them.1 The essential oil is made up of thujone, 1,8-cineole, camphor, borneol, isobutyl acetate, camphene, linalool, alpha- and beta-pinene, viridiflorol, alpa- and beta-caryphyllene. Sage blossoms in August. The flowers are labiate (lip-like) with a light purple, white, or pink color.2 Cultivation Sage grows well in almost all types of garden soil. It thrives in partial shade and warm, dryer soil. Though sage is a perennial, it usually needs to be replanted every few years due to the thinning of the plant.3 Origins The name scientific classification, salvia officinalis comes from the Latin verb salvare meaning to save. It was valued for its healing attributes as illustrated in a common Latin translation, “How can a man die who has Sage in his garden?” Some claim that the Virgin Mary used sage’s “extraordinary virtues” to guide her to Egypt and seek shelter.4 History The Ancients and Arabians considered sage linked to immortality. It was first found northern Mediterranean countries and eventually spread to England, France and Switzerland in the fourteenth century. -

Herbal Mixology : Bitters, Digestives and Aperitifs October 19, 2017
10/16/2017 Herbal Mixology : Bitters, Digestives and Aperitifs October 19, 2017 GLEN NAGEL, ND HERBALIST AND MIXOLOGIST [email protected] M.E.E.T The Herbs My herbal philosophy Medicine making is a medicine. Smoking Kava Drink Experience is the best teacher, make it something to remember and experience Everyday practice your craft, your art. Taste is the teacher, the new active ingredient is Taste, smell, sight. 1 10/16/2017 Herbal Mixology: The New Paradigm The problem with herbal medicine The problem with mixed drinks Taste is the active ingredient Alcohol as medicine? Organoleptics: the way of senses Herbs as medicine The Bitters Herbal Mixology Defined The power of herbal phytochemicals driven into the blood stream by alcohol and wrapped in an organoleptically rich sensual experience. This is the magic and power to Herbal Mixology. The art and science of adding medicinal value and action to the world of tasty alcoholic drinks Bringing the value of medical tonics back to the roots of botanical medicine My path as an herbalist, naturopathic doctor Making medicine is medicine, DIY 2 10/16/2017 The Problem with Herbal Medicine Tincture are alcoholic and water extracts sold as food extracts Growing industry of nutritional supplements, quality issues In general the problem as medicine is taste and compliance 90 percent of medicinal herbs taste bad to the average patient. Placing herbs in tablet or capsules gives less value, as the power is in the organoleptic experience. The Problem with Mixed Drinks or Cocktails Mixology history comes partially from herbal medicine and partially from pharmacy After the end of Prohibition there was increasing commercialization of alcohol distillation Increasing acceptance of mixed drinks with high alcohol content Increase in bars and speakeasy selling good times, and pushing high-alcohol, high-tastes drinks Lead to over consumption of sugar and alcohol, which lead to negative health effects. -

Therapeutic Uses of Peppermint –A Review
Aishwarya Balakrishnan /J. Pharm. Sci. & Res. Vol. 7(7), 2015, 474-476 Therapeutic Uses of Peppermint –A Review Aishwarya Balakrishnan, Saveetha Dental College,Chennai-77 Abstract: Peppermint (Mentha piperita, also known as M. balsamea Willd), is a hybrid mint, a cross between watermint and spearmint. The plant, indigenous to Europe and the Middle East, is now widespread in cultivation in many regions of the world. It is found wild occasionally with its parent species. The concentrated oil of peppermint has a high menthol content. The oil also contains menthone and menthyl esters, particularly menthyl acetate. Dried peppermint typically has volatile oil containing menthol, menthone , menthyl acetate ,menthofuran and 1,8-cineol. Peppermint oil also contains small amounts of many additional compounds including limonene, pulegone, caryophyllene and pinene. According to the German Commission E monographs, peppermint oil (as well as peppermint leaf) has been used internally as an antispasmodic (upper gastrointestinal tract and bile ducts) and to treat irritable bowel syndrome, catarrh of the respiratory tract, and inflammation of the oral mucosa. Externally, peppermint oil has been used for myalgia and neuralgia. According to Commission E, peppermint oil may also act as a carminative, cholagogue, antibacterial, and secretolytic, and it has a cooling action. Enteric-coated peppermint oil capsules (Colpermin) have been used as an orally administered antispasmodic premedication in colonoscopy. Key Words : Mentha piperita, peppermint, menthone. INTRODUCTION: important aromatic and medicinal crops produced in the Peppermint or mentha piperta is a common herb that is U.S. The world production of peppermint oil is about 8000 grown in Europe and north America. -

Spice Oleoresins
Institute of Medicine Food and Nutrition Board Committee on Food Chemicals Codex Revised Monograph - Spice Oleoresins Please send comments to the Committee on Food Chemicals Codex, National Academy of Sciences, FO 3042, 2101 Constitution Avenue, N.W., Washington, DC 20418 or email them to [email protected]. All comments must be received by December 15, 1996, for consideration for the First Supplement. ____________________________________________________________________________________ May 31, 1996 Spice Oleoresins DESCRIPTION Spice Oleoresins used in foods are derived from spices and contain the total sapid, odorous, and related characterizing principles normally associated with the respective spices. The oleoresins are produced by one of the following processes: (1) by extraction of the spice with any suitable solvent or solvents, in combination or sequence, followed by removal of the solvent or solvents in conformance with applicable residual solvent regulations (see General Requirements below), or (2) by removal of the volatile portion of the spice by distillation, followed by extraction of the nonvolatile portion, which after solvent removal is combined with the total volatile portion. Spice Oleoresins are frequently used in commerce with added suitable food-grade diluents, preservatives, antioxidants, and other substances consistent with good manufacturing practice, as provided for under Added Substances (see General Provisions). When added substances are used, they must be declared on the label in accordance with current U.S. regulations or with the regulations of other countries that recognize the Food Chemicals Codex. The Spice Oleoresins covered by this monograph are Oleoresin Angelica Seed Obtained by the solvent extraction of the dried seed of Angelica archangelica Linnaeus as a dark brown or green liquid. -

Bitters , Digestives and Aperitifs March 20Th 2017
Herbal Mixology : Bitters , Digestives and Aperitifs March 20th 2017 GLEN NAGEL, ND HERBALIST AND MIXOLOGIST [email protected] M.E.E.T The Herbs My herbal philosophy Medicine making is a medicine. Smoking Kava Drink Experience is the best teacher, make it something to remember and experience Everyday practice your craft, your art. Taste is the teacher, the new active ingredient is Taste, smell, sight. Herbal Mixology: The New Paradigm: Outline The problem with herbal medicine The problem with Mixed drinks Taste is the active ingredient Alcohol as medicine? Organoleptics: the way of senses Herbs as medicine The Bitters Herbal Mixology : Defined as The power of herbal phytochemicals driven into the blood stream by alcohol and wrapped in an organoleptically rich sensual experience: This is the magic and power to Herbal Mixology. The art and science of adding medicinal value and action to the world of tasty alcoholic drinks Bringing the value of medical tonics back to the roots of botanical medicine My path as an herbalist, naturopathic doctor Making medicine is medicine, DIY The problem with Herbal Medicine Tincture are alcoholic and water extracts sold as food extracts Growing industry of nutritional supplements, Quality issues In general the problem as medicine is taste and compliance 90 % of medicinal herbs taste bad to the average patient. Placing herbs in tablet or capsules gives less value as the power is in the organoleptic experience. The Problem with Mixed Drinks or Cocktail Mixology history comes from part herbal medicine and pharmacy After the end of probation there was the increasing commercialization of alcohol distillation Increasing acceptance of mixed drinks with high alcohol content Increase in bars and speakeasy selling good times, and pushing high alcohol , high tastes drinks Lead to over consumption of sugar and alcohol, which lead to negative health effects. -

List of Essential Oils with Their Health Benefits
List of Essential Oils Essential oils are used extensively in aromatherapy and various traditional medicinal systems. Due to the numerous health benefits of essential oils, they are being explored by the scientific community for treating a variety of diseases including cancer, HIV, asthma, bronchitis, heart strokes, etc. There are more than 90 essential oils each having its own health benefits. Every essential oil blends well with many other essential oils enabling herbalists prepare a number of aromatic preparations. Given below is a list of essential oils. Most of these oils are strong in nature and can cause side effects, if they are not taken in appropraite manner and quantities. Further, their benefits are indicative and therefore consult a medical practitioner before using these oils, internally or topically. In some cases, the benefits of the herb are also given, kindly click on a essential oil to know more about its health benefits. ---------------------------------------------------------------------------------------------------- --------------------- Allspice Essential Oil Properties: Anaesthetic, analgesic, anti oxidant, anti septic, carminative, relaxant, rubefacient, stimulant and tonic. Health Benefits: Induce numbness, pain relief, relaxes body & mind, brings redness in skin, stimulates functions ---------------------------------------------------------------------------------------------------- --------------------- Angelica Essential Oil Properties: Anti spasmodic, carminative, depurative, diaphoretic, digestive, diuretic, -

Essential Oils and Oleoresins Part 1
ABSTRACT This profile explores the production of oleoresins by Supercritical Fluid Extraction (SFE) in Trinidad. Oleoresins are non-volatile extracts which may be used for food flavours, aromatherapy products, nutraceuticals/ MANUFACTURING pharmaceuticals, perfumes and various other PROFILE 3A: ESSENTIAL uses such as security sprays, insecticides, dyes OILS AND OLEORESINS etc. PART 1 The Development of Project Profiles for the ENGINEERING INSTITUTE 2016 Manufacturing Sector of T&T The research contained within this document was commissioned by InvesTT Limited and conducted by the UWI, St. Augustine Campus Manufacturing Profile 3a: Essential Oils and Oleoresins Part 1 Table of Contents List of Tables .......................................................................................................................... iv List of Figures .......................................................................................................................... v 1 Description of the Opportunity ............................................................................................. 1 1.1 Summary ........................................................................................................................ 2 1.2 Product Mix.................................................................................................................... 4 1.3 Description of Activities ................................................................................................ 5 2 Industry Overview ............................................................................................................... -

Melissa Officinalis L., a Valuable Medicine Plant: a Review
Journal of Medicinal Plants Research Vol. 4(25), pp. 2753-2759, 29 December Special Review, 2010 Available online at http://www.academicjournals.org/JMPR ISSN 1996-0875 ©2010 Academic Journals Review Melissa officinalis L., a valuable medicine plant: A review Moradkhani H.1, Sargsyan E.1, Bibak H.2, Naseri B.3, Sadat-Hosseini M.2, Fayazi-Barjin A.4 and Meftahizade H.5* 1Institute of Hydroponic Problems, National Academic of Sciences, Yerevan, Republic of Armenia. 2Department of plant production, faculty of Agriculture, university of Jiroft, Kerman, Iran. 3Faculty of Islamic Azad University, Ilam, Iran. 4Department of Plant Protection, University of Tehran, Iran. 5Researcher of ACECR Medicinal Plants Center, Ilam, Iran. Accepted 6 December, 2010 Melissa officinalis L., a valuable medicinal plant in herbal medicine is native to the eastern Mediterranean Region and western Asia. The constituent of the essential oil of the plant in various climates is different, but citral (geranial and neral), citronellal, geraniol are main components. Many parameters influencing essential oil composition and yield, such as light intensity, nutrient, temperature, cultural practice genotype, plant part age, harvesting time. Lemon balm has been traditionally used for different medical purposes as tonic, antispasmodic, carminative, diaphoretic, surgical dressing for wounds, sedative-hypnotic strengthening the memory, and relief of stress induced headache, but in modern pharmacology is value in the management of mild to moderate Alzheimer’s, against migraine and rheumatism, antitumel and antioxidant activities. Key words: Melissa officinalis, essential oil, pharmacology and antioxidant. INTRODUCTION Lemon balm, member of the family Lamiaceae (formerly years may no longer germinate (Zargari, 1991). Labiatae) is a perennial bushy plant and is upright, Lemon balm has a hairy root system with many lateral reaching a height of about 1 m. -

Assessment Report on Salvia Officinalis L., Folium and Salvia Officinalis L., Aetheroleum Final
20 September 2016 EMA/HMPC/150801/2015 Committee on Herbal Medicinal Products (HMPC) Assessment report on Salvia officinalis L., folium and Salvia officinalis L., aetheroleum Final Based on Article 16d(1), Article 16f and Article 16h of Directive 2001/83/EC (traditional use) Herbal substance(s) (binomial scientific name of Salvia officinalis L., folium and the plant, including plant part) Salvia officinalis L., aetheroleum Herbal preparation(s) a) Comminuted herbal substance b) Liquid extract (DER 1:1), extraction solvent ethanol 70% V/V c) Dry extract (DER 4-7:1), extraction solvent water d) Liquid extract (DER 1:3.5-5), extraction solvent ethanol 31.5% V/V e) Liquid extract (DER 1:4-5) extraction solvent ethanol 50% V/V f) Liquid extract (DER 1:4-6), extraction solvent liquor wine:ethanol 96% V/V (38.25:61.75 m/m) g) Tincture (ratio of herbal substance to extraction solvent 1:10) extraction solvent ethanol 70% V/V Pharmaceutical form(s) Comminuted herbal substance as herbal tea for oral use. Comminuted herbal substance for infusion preparation for oromucosal or cutaneous use. Herbal preparations in solid or liquid dosage forms for oral use. Herbal preparations in liquid or semi-solid dosage forms for cutaneous use or for oromucosal use. 30 Churchill Place ● Canary Wharf ● London E14 5EU ● United Kingdom Telephone +44 (0)20 3660 6000 Facsimile +44 (0)20 3660 5555 Send a question via our website www.ema.europa.eu/contact An agency of the European Union © European Medicines Agency, 2017. Reproduction is authorised provided the source is acknowledged. -

Featuring Lemon Balm Herbal Water Lillian's
Recipes Featuring Lemon Balm Herbal Water 1 handful of lemon balm ½ handful of pineapple sage ½ handful orange mint ¼ handful mystery herb (like rose, lime basil, or rose scented geranium) in 2 quarts of water Six hours before drinking it, gather the herbs, wash them gently in cold water and place them in a jug of water. Place the jug in the fridge so that is refreshingly chilled for family or guests. For a morning garden tour, make this the night before. Any citrus, pleasant scented geranium, mint or other pleasing herbs can be substituted. © 2009 Lemon Balm: An Herb Society of America Guide, Recipe Karen Langan Lillian's Lemon Noodles 1 cup butter (no substitutions) Blend in: 1½ cups sugar 2¾ cups flour 2 eggs 1 teaspoon cream of tartar 6-8 leaves of lemon balm that have been 1 teaspoon baking soda finely chopped (can put in blender with Zest of one fresh lemon or a tablespoon eggs to be chopped) of dried grated lemon peel 1 teaspoon vanilla Chill batter 1 hour or longer. Roll small balls the size of a cherry or walnut depending on the size of cookie you like; bake at 350°F, 8-12 minutes, till golden. Batter can be kept up to a week covered, in refrigerator. While still warm, frost with small dollop of lemon butter icing. Lemon Butter Icing: Beat one stick of room temperature butter with 1 box 4X sugar. Add the juice of ½ lemon, 1 teaspoon vanilla; beat well. If too stiff, add a drop or two of milk.