Dermoscopy in General Dermatology (Non-Neoplastic Dermatoses) of Skin
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Melanocytes and Their Diseases
Downloaded from http://perspectivesinmedicine.cshlp.org/ on October 2, 2021 - Published by Cold Spring Harbor Laboratory Press Melanocytes and Their Diseases Yuji Yamaguchi1 and Vincent J. Hearing2 1Medical, AbbVie GK, Mita, Tokyo 108-6302, Japan 2Laboratory of Cell Biology, National Cancer Institute, National Institutes of Health, Bethesda, Maryland 20892 Correspondence: [email protected] Human melanocytes are distributed not only in the epidermis and in hair follicles but also in mucosa, cochlea (ear), iris (eye), and mesencephalon (brain) among other tissues. Melano- cytes, which are derived from the neural crest, are unique in that they produce eu-/pheo- melanin pigments in unique membrane-bound organelles termed melanosomes, which can be divided into four stages depending on their degree of maturation. Pigmentation production is determined by three distinct elements: enzymes involved in melanin synthesis, proteins required for melanosome structure, and proteins required for their trafficking and distribution. Many genes are involved in regulating pigmentation at various levels, and mutations in many of them cause pigmentary disorders, which can be classified into three types: hyperpigmen- tation (including melasma), hypopigmentation (including oculocutaneous albinism [OCA]), and mixed hyper-/hypopigmentation (including dyschromatosis symmetrica hereditaria). We briefly review vitiligo as a representative of an acquired hypopigmentation disorder. igments that determine human skin colors somes can be divided into four stages depend- Pinclude melanin, hemoglobin (red), hemo- ing on their degree of maturation. Early mela- siderin (brown), carotene (yellow), and bilin nosomes, especially stage I melanosomes, are (yellow). Among those, melanins play key roles similar to lysosomes whereas late melanosomes in determining human skin (and hair) pigmen- contain a structured matrix and highly dense tation. -

Uniform Faint Reticulate Pigment Network - a Dermoscopic Hallmark of Nevus Depigmentosus
Our Dermatology Online Letter to the Editor UUniformniform ffaintaint rreticulateeticulate ppigmentigment nnetworketwork - A ddermoscopicermoscopic hhallmarkallmark ooff nnevusevus ddepigmentosusepigmentosus Surit Malakar1, Samipa Samir Mukherjee2,3, Subrata Malakar3 11st Year Post graduate, Department of Dermatology, SUM Hospital Bhubaneshwar, India, 2Department of Dermatology, Cloud nine Hospital, Bangalore, India, 3Department of Dermatology, Rita Skin Foundation, Kolkata, India Corresponding author: Dr. Samipa Samir Mukherjee, E-mail: [email protected] Sir, ND is a form of cutaneous mosaicism with functionally defective melanocytes and abnormal melanosomes. Nevus depigmentosus (ND) is a localized Histopathologic examination shows normal to hypopigmentation which most of the time is congenital decreased number of melanocytes with S-100 stain and and not uncommonly a diagnostic challenge. ND lesions less reactivity with 3,4-dihydroxyphenylalanine reaction are sometimes difficult to differentiate from other and no melanin incontinence [2]. Electron microscopic hypopigmented lesions like vitiligo, ash leaf macules and findings show stubby dendrites of melanocytes nevus anemicus. Among these naevus depigmentosus containing autophagosomes with aggregates of poses maximum difficulty in differentiating from ash melanosomes. leaf macules because of clinical as well as histological similarities [1]. Although the evolution of newer diagnostic For ease of understanding the pigmentary network techniques like dermoscopy has obviated the -

Skin Pigmentary Variants in Rana Nigromaculata
Original Article J. Clin. Biochem. Nutr., 38, 195–203, May 2006 Skin Pigmentary Variants in Rana Nigromaculata Ichiro Tazawa, Hitoshi Okumoto, and Akihiko Kashiwagi* Division of Embryology and Genetics, Institute for Amphibian Biology, Graduate School of Science, Hiroshima University, 1-3-1 Kagamiyama, Higashihiroshima, Hiroshima 739-8526, Japan Received 22 December, 2005; Accepted 26 January, 2006 Summary Because there is mounting evidence to suggest that oxidative stress is involved in the pathophysiology of albinism, albino amphibians are useful tools for studies on imbalances in the oxidant-antioxidant system. In the course of maintaining albino mutant frog strains it was found that crosses between albino males and heterozygous females of Rana nigromaculata sometimes produce offspring displaying pigmentary mosaicism. After hatching hypopigmented portions appear on the left or right side of the body, and this is accompanied by such abnormalities as poor viability, asymmetrical curvature of the body toward the hypopigmented side, and limb deformity. Histological examination of mosaics showed the cells of various tissues (except kidney) to be smaller on the hypopigmented side and larger on the pigmented side compared to corresponding cells in wild type offspring. Cytogenetic analysis of cultured skin cells revealed that wild type and albino individuals were diploidal with 26 chromosomes, the same as normal R. nigromaculata. In mosaics on the other hand, cells of hypopigmented portions were almost exclusively haploidal with 13 chromosomes, while pigmented portions were a mixture of roughly 75% triploidal, 39 chromosome cells and roughly 25% haploidal, 13 chromosome cells. Key Words: albino, unilateral pigmentation, pigmentary mosaicism, chromosomal mosaicism, mixoploidy, haploid, triploid, Rana one means of assessing the involvement of reactive oxygen Introduction species (ROS) in biological processes such as aging. -

Frequency of Different Types of Facial Melanoses Referring to the Department of Dermatology and Venereology, Nepal Medical Colle
Amatya et al. BMC Dermatology (2020) 20:4 https://doi.org/10.1186/s12895-020-00100-3 RESEARCH ARTICLE Open Access Frequency of different types of facial melanoses referring to the Department of Dermatology and Venereology, Nepal Medical College and Teaching Hospital in 2019, and assessment of their effect on health-related quality of life Bibush Amatya* , Anil Kumar Jha and Shristi Shrestha Abstract Background: Abnormalities of facial pigmentation, or facial melanoses, are a common presenting complaint in Nepal and are the result of a diverse range of conditions. Objectives: The objective of this study was to determine the frequency, underlying cause and impact on quality of life of facial pigmentary disorders among patients visiting the Department of Dermatology and Venereology, Nepal Medical College and Teaching Hospital (NMCTH) over the course of one year. Methods: This was a cross-sectional study conducted at the Department of Dermatology and Venereology, NMCT H. We recruited patients with facial melanoses above 16 years of age who presented to the outpatient department. Clinical and demographic data were collected and all the enrolled participants completed the validated Nepali version of the Dermatology Life Quality Index (DLQI). Results: Between January 5, 2019 to January 4, 2020, a total of 485 patients were recruited in the study. The most common diagnoses were melasma (166 patients) and post acne hyperpigmentation (71 patients). Quality of life impairment was highest in patients having melasma with steroid induced rosacea-like dermatitis (DLQI = 13.54 ± 1.30), while it was lowest in participants with ephelides (2.45 ± 1.23). Conclusion: Facial melanoses are a common presenting complaint and lead to substantial impacts on quality of life. -

Colocalized Nevus Depigmentosus and Lentigines Prashansa Jaiswal, Sundeep Chowdhry, Paschal D’ Souza
2XU'HUPDWRORJ\2QOLQH Case Report Colocalized nevus depigmentosus and lentigines Prashansa Jaiswal, Sundeep Chowdhry, Paschal D’ Souza Department of Dermatology, Venereology and Leprology, Employees’ State Insurance Corporation Post Graduate Institute of Medical Sciences & Research, Basaidarapur, New Delhi - 110 015, India Corresponding author: Assist. Prof. Sundeep Chowdhry, E-mail: [email protected] ABSTRACT Nevus depigmentosus (ND)is classically defined as a congenital nonprogressive hypopigmented macule, stable in size and distribution. A 17 year girl presented with hypopigmented patch with indented borders, present on the right side of face and neck since 3 years of age. Later on at the age of 5, numerous hyperpigmented punctiform spots appeared exclusively on the hyperpigmented area. On sun exposure, the hypopigmented area neither reddened nor burnt. On diascopy the margin of the hypopigmented lesion remained delineated. The dermoscopic examination showed 1-4 millimeters sized hyperpigmented lesions with a barely visible pseudonet, leading to the final diagnosis of colocalized nevus depigmentosus and lentigines. Key words: Nevus; Hypopigmentation; Reverse; Mutation; Pigmentation INTRODUCTION 25 X 8 centimeters was present at the angle of mouth on right side, further extending to lateral side of right ear, Nevus depigmentosus (ND)is a rare, congenital, right angle of jaw, lateral right side of neck to about 6 stable hypomelanosis first described by Lesser in centimeters below the clavicle. It was irregular in shape 1884 [1]. The lesions usually present as dermatomal with serrated irregular margins. The surface was smooth or quasidermatomal macules commonly on the trunk, and had multiple oval dark brown coloured macules of lower abdomen, or proximal extremities. They are off- 1 to 4 mm in size (Fig. -

Melasma on the Nape of the Neck in a Man
Letters to the Editor 181 Melasma on the Nape of the Neck in a Man Ann A. Lonsdale-Eccles and J. A. A. Langtry Sunderland Royal Hospital, Kayll Road, Sunderland SR4 7TP, UK. E-mail: [email protected] Accepted July 19, 2004. Sir, sunlight and photosensitizing agents may be more We report a 47-year-old man with light brown macular relevant. pigmentation on the nape of his neck (Fig. 1). It was The differential diagnosis for pigmentation at this site asymptomatic and had developed gradually over 2 years. includes Riehl’s melanosis, Berloque dermatitis and He worked outdoors as a pipe fitter on an oilrig module; poikiloderma of Civatte. Riehl’s melanosis typically however, he denied exposure at this site because he involves the face with a brownish-grey pigmentation; always wore a shirt with a collar that covered the biopsy might be expected to show interface change and affected area. However, on further questioning it liquefaction basal cell degeneration with a moderate transpired that he spent most of the day with his head lymphohistiocytic infiltrate, melanophages and pigmen- bent forward. This reproducibly exposed the area of tary incontinence in the upper dermis. It is usually pigmentation with a sharp cut off inferiorly at the level associated with cosmetic use and may be considered of his collar. He used various shampoos, aftershaves and synonymous with pigmented allergic contact dermatitis shower gels, but none was applied directly to that area. of the face (6, 7). Berloque dermatitis is considered to be His skin was otherwise normal and there was no family caused by a photoirritant reaction to bergapentin; it history of abnormal pigmentation. -

Phacomatosis Spilorosea Versus Phacomatosis Melanorosea
Acta Dermatovenerologica 2021;30:27-30 Acta Dermatovenerol APA Alpina, Pannonica et Adriatica doi: 10.15570/actaapa.2021.6 Phacomatosis spilorosea versus phacomatosis melanorosea: a critical reappraisal of the worldwide literature with updated classification of phacomatosis pigmentovascularis Daniele Torchia1 ✉ 1Department of Dermatology, James Paget University Hospital, Gorleston-on-Sea, United Kingdom. Abstract Introduction: Phacomatosis pigmentovascularis is a term encompassing a group of disorders characterized by the coexistence of a segmental pigmented nevus of melanocytic origin and segmental capillary nevus. Over the past decades, confusion over the names and definitions of phacomatosis spilorosea, phacomatosis melanorosea, and their defining nevi, as well as of unclassifi- able phacomatosis pigmentovascularis cases, has led to several misplaced diagnoses in published cases. Methods: A systematic and critical review of the worldwide literature on phacomatosis spilorosea and phacomatosis melanorosea was carried out. Results: This study yielded 18 definite instances of phacomatosis spilorosea and 14 of phacomatosis melanorosea, with one and six previously unrecognized cases, respectively. Conclusions: Phacomatosis spilorosea predominantly involves the musculoskeletal system and can be complicated by neuro- logical manifestations. Phacomatosis melanorosea is sometimes associated with ancillary cutaneous lesions, displays a relevant association with vascular malformations of the brain, and in general appears to be a less severe syndrome. -

An Unusual Presentation of a Unilateral Asymptomatic Riehl's
ts & C por a e se R S l Michael, Med Rep Case Stud 2018, 3:1 t a u c d i i DOI: 10.4172/2572-5130.1000152 d e s e M + Medical Reports and Case Studies ISSN: 2572-5130 Case Report Open Access An Unusual Presentation of a Unilateral Asymptomatic Riehl’s Melanosis in a 45 Year Old Male Chan Kam Tim Michael* Department of Dermatology, Hong Kong Academy of Medicine, Hong Kong *Corresponding author: Chan Kam Tim Michael, Department of Dermatology, Hong Kong Academy of Medicine, Hong Kong, Tel: +85221282129; E-mail: [email protected] Received Date: Feb 12, 2018; Accepted Date: Mar 12, 2018; Published Date: Mar 21, 2018 Copyright: © 2018 Michael CKT. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Introduction but of post-inflammatory hyperpigmentation, Acquired unilateral Nevus (Hori’s Nevus), Riehl’s melanosis, Drug-induced Pigmented contact dermatitis (PCD), also known as Riehl’s hyperpigmentation, Lichenoid dermatitis; as well as Melasma and melanosis, is a rare facial hyperpigmentation usually secondary to Ochronosis. cosmetics. There are few documented reports in the literature, and many cases without proven diagnosis may have been treated with pigment lasers, especially in beauty parlour settings. We report a case referred from a private practitioner who has a special interest in dermatology. The patient was diagnosed subsequently as having Riehl’s melanosis and treated with non-tyrosinase inhibitor bleaching agents, sun avoidance and mandatory abstinence from over-the-counter cosmetic products. -

Preceded by Multiple Diagnostic. X-Rays One Year Prior to The
A Malformation Complex of Ectrodactyly, Clefting and Hypomelanosis of Ito (Incontinentia Pigmenti Achromians) RAY E. STEWART, D.M.D., M.S. STEVEN FUNDERBURK, M.D. YOSHIO SETOGUCHI, M.D. Torrance, California 90509 A case is described which, at birth, had a bizarre pattern of Aypopigmentation (incontinentia pigment achromians), ectrodactyly involving all four extremities, and unilateral cleft lip and palate. This patient does not have the seizures or other neurological and developmental anomalies previously described as associated with Aypopigmentation of Ito. This condition is also clearly different from the syndrome of ectrodactyly, ectodermal dysplasia, and clefting (EEC). We have recently observed four-month-old tone. The child has developed normally, Mexican-American female infant with an in- reaching all of her age-appropriate milestones teresting constellation of anomalies, including with no evidence of neurological or other _ unilateral complete clefts of the lip and pal- problems. The Denver Developmental Scales ate, ectrodactyly of the hands and feet, and yielded an intelligence quotient of 100 at 14 generalized abnormalities in skin pigmenta- months of age. tion. The association of ectodermal changes, Examination of the head and neck revealed ectrodactyly, and oralfacial clefting has been unilateral complete cleft of the lip and palate. well documented in the EEC syndrome (ec- There was mild hypoplasia of the right exter- trodactyly, ectodermal dysplasia, and clefting nal ear (Figure 1). syndrome). However, this patient clearly does Examination of the skin revealed a peculiar not fall into this classification. generalized pattern of hypopigmentation over the entire body, including the head, neck, and Clinical findings face. On the dorsum, the pigmentation as- The child was the product of a full-term, sumed a swirling pattern. -

Nevus Depigmentosus
Journal of Dermatology & Cosmetology Commentary Open Access Nevus depigmentosus Introduction Volume 1 Issue 1 - 2017 Nevus Depigmentosus (nevus achromicus) is a rare congenital pigmentary disorder. It is a depigmentation problem in skin which can Hayk S Arakelyan be easily differentiated from vitiligo. Nevus anemic us is a congenital General Medicine and Clinical Research, Armenian University of vascular anomaly that presents clinically as a hypo pigmented macule Integrative Medicine, Armenia or patch. Correspondence: Hayk S Arakelyan, Doctor of Medical The pathogenesis of ND is not fully understood. It is believed Sciences, PhD, Senior Expert of Interactive Clinical to be due to a functional defect of melanocytes with morphological Pharmacology, Drug Safety, Treatment Tactics, General Medicine abnormalities of melanosomes. It is also said to be a form of cutaneous and Clinical Research. Armenian University of Integrative mosaicism wherein an altered clone of melanocyte have a decreased Medicine, Armenia, Tel (37491)-40-94-97, Email [email protected] ability to synthesize melanin and transport to keratinocytes. Received: March 28, 2017 | Published: April 04, 2017 It can be found anywhere on the body but commonly it is seen on the trunk, neck, face, and proximal part of the extremities. Clinically, three types have been described: Localized Segmental, and Linear or Whorled. Localized variant is the most common compared to the others. It is a single well circumscribed lesion with serrated Acknowledgements borders. Segmental variant is larger in size and shape and also referred to as “segmental de-pigmentation disorder” with a sharp midline None. demarcation. Linear/whorled/systematized type may be extensive and have cutaneous lesions that overlap with HOI.( hypomelanosis of Ito ) Conflict of interest The systematized variant is very rare and may have extra cutaneous The author declares no conflict of interest. -
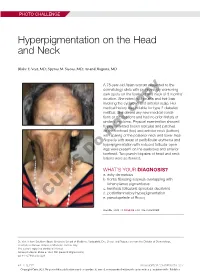
Hyperpigmentation on the Head and Neck
PHOTO CHALLENGE Hyperpigmentation on the Head and Neck Blake E. Vest, MD; Spyros M. Siscos, MD; Anand Rajpara, MD A 78-year-old Asian woman presented to the dermatology clinic with progressively worsening dark spots on the forehead and neck of 3 months’ duration. She noted mild pruritis and hair loss involving the eyebrows and anterior scalp. Her medical history was notable for type 2 diabetes mellitus. She deniedcopy any new medical condi- tions or medications and had no prior history of similar symptoms. Physical examination showed hyperpigmented brown macules and patches on the forehead (top) and anterior neck (bottom) withnot sparing of the posterior neck and lower face. Alopecia with areas of perifollicular erythema and hyperpigmentation with reduced follicular open- ings were present on the eyebrows and anterior Doforehead. Two punch biopsies of head and neck lesions were performed. WHAT’S YOUR DIAGNOSIS? a. ashy dermatosis b. frontal fibrosing alopecia overlapping with lichen planus pigmentosus c. keratosis follicularis spinulosa decalvans d. postinflammatory hyperpigmentation CUTIS e. pseudopelade of Brocq PLEASE TURN TO PAGE E3 FOR THE DIAGNOSIS Dr. Vest is from Southern Illinois University School of Medicine, Springfield. Drs. Siscos and Rajpara are from the Division of Dermatology, University of Kansas School of Medicine, Kansas City. The authors report no conflict of interest. Correspondence: Blake E. Vest, MD ([email protected]). doi:10.12788/cutis.0226 E2 I CUTIS® WWW.MDEDGE.COM/DERMATOLOGY Copyright Cutis 2021. No part of this publication may be reproduced, stored, or transmitted without the prior written permission of the Publisher. PHOTO CHALLENGE DISCUSSION THE DIAGNOSIS: Frontal Fibrosing Alopecia Overlapping With Lichen Planus Pigmentosus icroscopic examination revealed focal dermal of the skin have been reported, including the entire pigmentation, papillary fibrosis, and epidermal scalp, eyebrows, and eyelashes. -

Pigmented Contact Dermatitis and Chemical Depigmentation
18_319_334* 05.11.2005 10:30 Uhr Seite 319 Chapter 18 Pigmented Contact Dermatitis 18 and Chemical Depigmentation Hideo Nakayama Contents ca, often occurs without showing any positive mani- 18.1 Hyperpigmentation Associated festations of dermatitis such as marked erythema, with Contact Dermatitis . 319 vesiculation, swelling, papules, rough skin or scaling. 18.1.1 Classification . 319 Therefore, patients may complain only of a pigmen- 18.1.2 Pigmented Contact Dermatitis . 320 tary disorder, even though the disease is entirely the 18.1.2.1 History and Causative Agents . 320 result of allergic contact dermatitis. Hyperpigmenta- 18.1.2.2 Differential Diagnosis . 323 tion caused by incontinentia pigmenti histologica 18.1.2.3 Prevention and Treatment . 323 has often been called a lichenoid reaction, since the 18.1.3 Pigmented Cosmetic Dermatitis . 324 presence of basal liquefaction degeneration, the ac- 18.1.3.1 Signs . 324 cumulation of melanin pigment, and the mononucle- 18.1.3.2 Causative Allergens . 325 ar cell infiltrate in the upper dermis are very similar 18.1.3.3 Treatment . 326 to the histopathological manifestations of lichen pla- 18.1.4 Purpuric Dermatitis . 328 nus. However, compared with typical lichen planus, 18.1.5 “Dirty Neck” of Atopic Eczema . 329 hyperkeratosis is usually milder, hypergranulosis 18.2 Depigmentation from Contact and saw-tooth-shape acanthosis are lacking, hyaline with Chemicals . 330 bodies are hardly seen, and the band-like massive in- 18.2.1 Mechanism of Leukoderma filtration with lymphocytes and histiocytes is lack- due to Chemicals . 330 ing. 18.2.2 Contact Leukoderma Caused Mainly by Contact Sensitization .