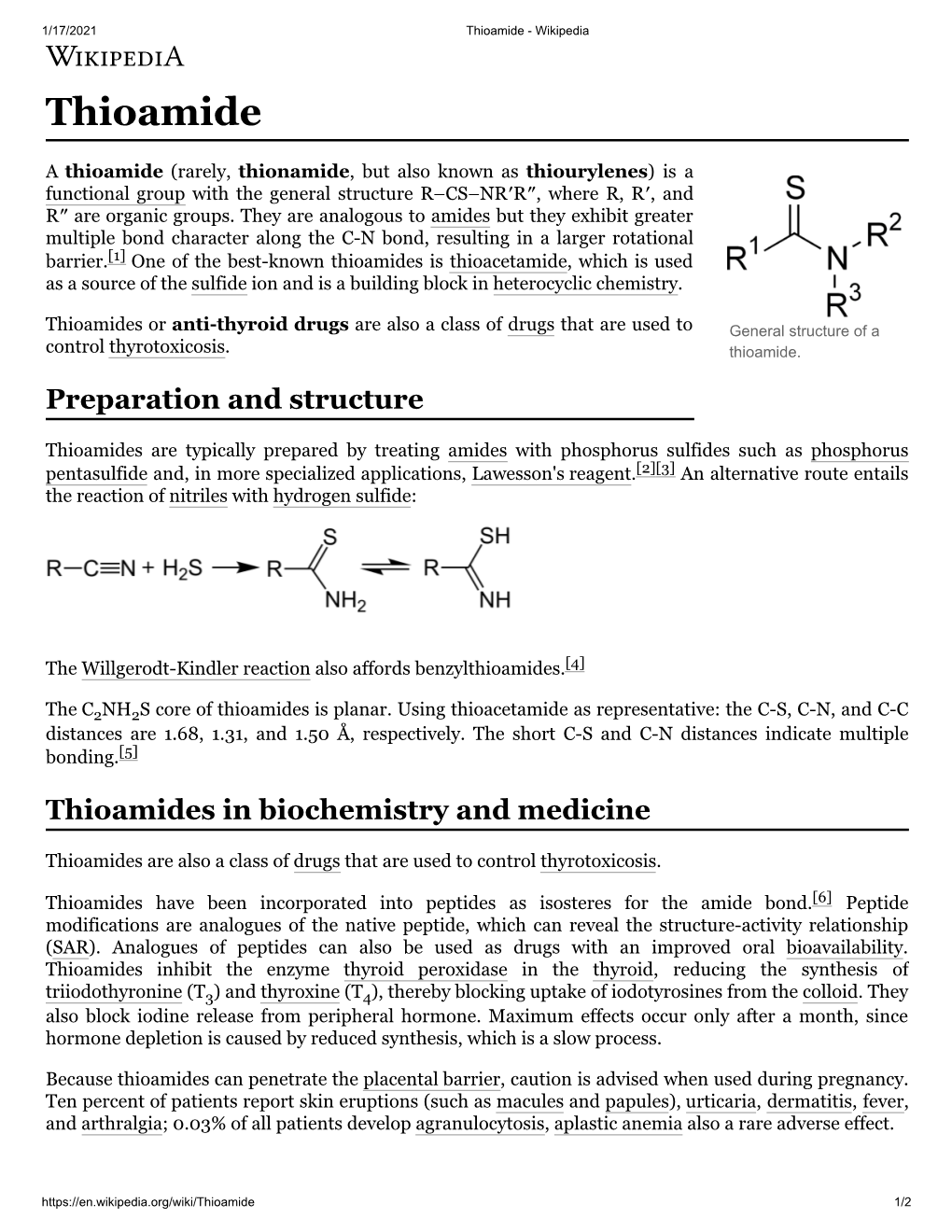
Thioamide - Wikipedia
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

PRODUCT INFORMATION Propylthiouracil-D5 Item No
PRODUCT INFORMATION Propylthiouracil-d5 Item No. 30743 CAS Registry No.: 1189423-94-6 Formal Name: 2,3-dihydro-6-(propyl-2,2,3,3,3-d5)-2- thioxo-4(1H)-pyrimidinone S Synonyms: 6-n-Propylthiouracil-d5, PTU-d5 MF: C H D N OS H H 7 5 5 2 N N FW: 175.3 D D Chemical Purity: ≥98% (Propylthiouracil) D O Deuterium D Incorporation: ≥99% deuterated forms (d1-d5); ≤1% d0 D Supplied as: A solid Storage: -20°C Stability: ≥2 years Information represents the product specifications. Batch specific analytical results are provided on each certificate of analysis. Laboratory Procedures Propylthiouracil-d5 is intended for use as an internal standard for the quantification of propylthiouracil (Item No. 14069) by GC- or LC-MS. The accuracy of the sample weight in this vial is between 5% over and 2% under the amount shown on the vial. If better precision is required, the deuterated standard should be quantitated against a more precisely weighed unlabeled standard by constructing a standard curve of peak intensity ratios (deuterated versus unlabeled). Propylthiouracil-d5 is supplied as a solid. A stock solution may be made by dissolving the propylthiouracil-d5 in the solvent of choice, which should be purged with an inert gas. Propylthiouracil-d5 is slightly soluble in DMSO and methanol. Description Propylthiouracil (PTU) is a thioamide antithyroid agent.1 It inhibits thyroid peroxidase activity in rat and monkey thyroid microsomes (IC50s = 0.081 and 4.1 μM, respectively). PTU (30 mg/kg) increases thyroid weight and serum thyroid stimulating hormone levels and decreases serum 3,5,3’-triiodothyronine and thyroxine levels in rats. -

Chapter 38 Thyroid & Antithyroid Drugs
Chapter 38 Thyroid & antithyroid drugs Right Lobe Left Lobe Thyroglobulin Thyroid gland Structure: • The thyroid gland consists of two lobes & is situated in the lower neck • The functional unit of the thyroid gland is the follicle • Each follicle consists of a single layer of epithelial cells (follicular cells) around a cavity, >>> • the follicle lumen, which is filled with a thick colloid containing thyroglobulin – Thyroglobulin is a large glycoprotein, – each molecule of which contains about 115 tyrosine residues Thyroid homones • The thyroid gland secretes: triiodothyronine (T3) thyroxine (T4)…iodothyronines • The thyroid gland also secretes calcitonin • T3 and T4 are critically important for: – normal growth and development, – normalize body temp. – normalize energy levels • Calcitonin is involved in the control of plasma Ca2+ (Ch. 42) Synthesis, storage, release, and interconversion of thyroid hormones Iodine intake Iodide is ingested by food, water, medication… rapidly absorbed (best absorbed in the duodenum and ileum) The daily intake is: 150mcg (200mcg during pregnancy) The thyroid gland removes 75mcg daily Synthesis, storage, release, and interconversion of thyroid hormones 1) Iodide ion (I-) is uptaken by the follicular cell (Na/I- symporter)…that incorporate it into active thyroid hormone. 2) Iodide is oxidized by thyroidal peroxidase into iodine 3) Iodine iodinates tyrosine residues of thyroglobulin to form monoiodotyrosine (MIT) and diiodotyrosine (DIT)….iodine organification 4) Two molecules of diiodotyrosine combine -

Carbimazole-Induced Hepatotoxicity - Avoid Rechallenging
Central JSM Thyroid Disorders and Management Bringing Excellence in Open Access Case Report *Corresponding author Marianne Elston, Department of Endocrinology, Waikato Hospital, Private Bag 3200, Hamilton 3240, New Carbimazole-Induced Zealand, Tel: +647 839 8899; Email: Submitted: 01 August 2016 Hepatotoxicity - Avoid Accepted: 10 August 2016 Published: 11 August 2016 Rechallenging Copyright © 2016 Elston et al. Karalus M, Jade Tamatea AU, Conaglen JV, and Elston MS* Waikato Clinical Campus, University of Auckland, New Zealand OPEN ACCESS Keywords Abstract • Antithyroid agents All thioamide anti - thyroid medications are known to be associated with liver • Drug-induced liver injury dysfunction. Cholestasis is most commonly reported in association with carbimazole/ • Thyrotoxicosis methimazole. We report a patient with Graves’ disease, who developed acute • Graves’s disease Hepatocellular dysfunction associated with carbimazole. There is limited data available on re -exposure in this situation. The patient rechallenged herself twice and subsequent exposures resulted in an increasingly shorter time to symptom onset until finally even a single 5mg tablet rapidly provoked severe symptoms and abnormal liver function tests. This case supports the need to avoid anti - thyroid medication rechallenge in patients who develop significant liver dysfunction following their use and identifies that carbimazole may be associated with acute Hepatocellular dysfunction and not just Cholestasis. ABBREVIATIONS thyrotoxicosis with normal liver function tests (LFTs) (Figure 1 and Table 1). Initial therapy with 20mg CBZ once daily was ATD: Anti - Thyroid Drugs; PTU: Propylthiouracil; CBZ: reduced after 2 weeks to 10mg per day. One month after starting Carbimazole; MMZ: Methimazole; ALT: Alanine Transaminase CBZ, routine liver function testing revealed abnormal LFTs INTRODUCTION particularly alanine transaminase (ALT) (Figure 1 and Table 1). -

JPBMAL2832.Pdf
B. Kumar, JPBMAL, 2016, 4(2): 122-131 CODEN (USA): JPBAC9 | ISSN: 2347-4742 Journal of Pharmaceutical and Biomedical Analysis Letters Journal Home Page: www.pharmaresearchlibrary.com/jpbmal Review Article Open Access A Review on Thyroid Disease B. Kumar*, K. Durga Prasanna Roja, M. Gobinath Department of Pharmacy Practice, Ratnam Institute of Pharmacy, Pidthapolur, Nellore. A B S T R A C T The thyroid gland is one of the largest endocrine gland and consists of two connected lobes. The thyroid gland is found in the neck, below the thyroid cartilage. It participates in these processes by producing thyroid hormones, the principal ones being triiodothyronine (T3) and thyroxine (sometimes referred to as tetraiodothyronine (T4)). These hormones regulate the growth and rate of function of many other systems in the body. T3 and T4 are synthesized from iodine and tyrosine. The primary function of the thyroid is production of the hormones T3, T4 and calcitonin. Up to 80% of the T4 is converted to T3 by organs such as the liver, kidney and spleen. T3 is several times more powerful than T4, which is largely a prohormone, perhaps four or even ten times more active. Beta-blockers are used to decrease symptoms of hyperthyroidism such as increased heart rate, tremors, anxiety and heart palpitations, and anti-thyroid drugs are used to decrease the production of thyroid hormones, in particular, in the case of Graves' disease. The gland shrinks by 50-60% but can cause hypothyroidism and rarely pain syndrome, which arises due to radiation thyroiditis. It is short lived and treated by steroids. -

Thyrotoxic Hypokalaemic Periodic Paralysis
Thyrotoxic Hypokalaemic Periodic Paralysis: A Case Series from Trinidad, West Indies M Ramdath1, J Teelucksingh1, A Ramnath1, K Ramcharan2, L Conyette2, A Ramesar2, RP King3, S Teelucksingh4 ABSTRACT We report our experience with seven cases of thyrotoxicosis associated with hypokalaemic periodic paralysis seen over a 25-year period in Trinidad, West Indies, an ethnically diverse island. All the cases were males, one was a Chinese, two were of East Indian and four of African descent, with a mean age 31.4 years (range 22–54 years). Early diagnosis and appropriate treatment led to the prompt resolution of the symptoms. Although rare in the Caribbean region, clinicians should be aware of the features of this potentially fatal but readily remediable com- plication of thyrotoxicosis. Keywords: Hypokalaemia, hypokalaemic periodic paralysis, periodic paralysis, thyrotoxic periodic paralysis, Trinidad and Tobago, West Indies Parálisis Periódica Hipocalémica Tirotóxica: Un Estudio de Serie de Casos de Trinidad, West Indies M Ramdath1, J Teelucksingh1, A Ramnath1, K Ramcharan2, L Conyette2, A Ramesar2, RP King3, S Teelucksingh4 RESUMEN Reportamos nuestra experiencia con siete casos de tirotoxicosis asociada con parálisis perió- dica hipocalémica observada por un período de 25 años en Trinidad, West Indies – una isla ca- racterizada por su diversidad étnica. En todos los casos se trató de hombres: uno era un chino, dos eran de la India, y cuatro de ascendencia africana, con una edad promedio de 31.4 años (rango 22 a 54 años). El diagnóstico precoz y un tratamiento adecuado llevaron a la pronta re- solución de los síntomas. Aunque sea raro en la región del Caribe, los clínicos deben tener en cuenta que las características de esta complicación de la tirotoxicosis, potencialmente fatal pero fácilmente remediable. -

Thyroidectomy for the Treatment of Graves' Thyrotoxicosis in Thioamide- Induced Agranulocytosis and Sepsis
Thyroidectomy for the treatment of Graves' thyrotoxicosis in thioamide- induced agranulocytosis and sepsis Citation: Knight, Colin L., Cooray, Shamil D., Kulkarni, Jaideep, Borschmann, Michael and Kotowicz, Mark 2017, Thyroidectomy for the treatment of Graves' thyrotoxicosis in thioamide- induced agranulocytosis and sepsis, Endocrinology, diabetes & metabolism, Article ID: 17- 0071, pp. 1-6. DOI: http://www.dx.doi.org/10.1530/EDM-17-0071 © 2017, The Authors Reproduced by Deakin University under the terms of the Creative Commons Attribution Non- Commercial No-Derivatives Licence Downloaded from DRO: http://hdl.handle.net/10536/DRO/DU:30109157 DRO Deakin Research Online, Deakin University’s Research Repository Deakin University CRICOS Provider Code: 00113B ID: 17-0071 10.1530/EDM-17-0071 C L Knight and others Thyroidectomy when thyrotoxic ID: 17-0071; September 2017 with agranulocytosis DOI: 10.1530/EDM-17-0071 Thyroidectomy for the treatment of Graves’ thyrotoxicosis in thioamide-induced agranulocytosis and sepsis Colin L Knight1, Shamil D Cooray1, Jaideep Kulkarni1, Michael Borschmann2,3 and Mark Kotowicz1,4,5 1Department of Endocrinology and Diabetes, University Hospital Geelong, Geelong, Victoria, Australia, Correspondence 2Ear, Nose and Throat, Head and Neck Surgery, St. John of God Hospital, Geelong, Victoria, Australia, should be addressed 3Director of Otolaryngology, University Hospital Geelong, Geelong, Victoria, Australia, 4Deakin University to C Knight School of Medicine, Geelong, Victoria, Australia, and 5Melbourne Clinical School-Western Campus, Email Department of Medicine, The University of Melbourne, St. Albans, Victoria, Australia [email protected] Summary A 51 year old man presented with sepsis in the setting of thioamide-induced agranulocytosis. Empiric broad-spectrum antibiotics was followed by directed narrow-spectrum antibiotics, and his neutrophil count recovered with support from granulocyte-colony stimulating factor (G-CSF) analogue transfusions. -

Thyroid Disease Testing AHS – G2045
Corporate Medical Policy Thyroid Disease Testing AHS – G2045 File Name: thyroid_disease_testing Origination: 4/2019 Last CAP Review: 6/2021 Next CAP Review: 6/2022 Last Review: 6/2021 Description of Procedure or Service Definition Thyroid hormones are necessary for prenatal and postnatal development, as well as metabolic activity in adults (Brent, 2020). Thyroid disease includes conditions which cause hypothyroidism, hyperthyroidism, goiter, thyroiditis (which can present as either hypo- or hyper-thyroidism) a nd thyroid tumors (Rugge, Bougatsos, & Chou, 2015). Thyroid function tests are used in a variety of clinical settings to assess thyroid function, monitor treatment, and screen asymptomatic populations for subclinical or otherwise undiagnosed thyroid dysfunction (Ross, 2019c). Related Policies: Genetic Cancer Susceptibility Using Next Generation Sequencing Genetic Testing for Germline Mutations of the RET Proto-Oncogene Molecular Markers in Fine Needle Aspirates of the Thyroid Molecular Panel Testing of Cancers to Identify Targeted Therapy Prenatal Screening ***Note: This Medical Policy is complex and technical. For questions concerning the technical language and/or specific clinical indications for its use, please consult your physician. Policy BCBSNC will provide coverage for thyroid disease testing when it is determined the medical criteria or reimbursement guidelines below are met Benefits Application This medical policy relates only to the services or supplies described herein. Please refer to the Member's Benefit Booklet for availability of benefits. Member's benefits may vary according to benefit design; therefore member benefit language should be reviewed before applying the terms of this medical policy. When thyroid disease testing is covered 1) Reimbursement for thyroid function testing is a llowed in the following situations: A. -

PTU™ 50 Mg Tablets
PRODUCT INFORMATION PTU™ 50 mg Tablets PTU™ 50 mg Tablets NAME OF THE MEDICINE Propylthiouracil Chemical Name: 1,2-dihydro-6-propyl-2-thioxopyrimidine-4-one The molecular weight of the compound is 170.2, the molecular formula is C 7H10 N2OS and the CAS registry number is 51-52-5. Structural Formula : DESCRIPTION Propythiouracil is a thioamide derivative which occurs as a white crystalline powder, odourless with a bitter taste, very slightly soluble in water, sparingly soluble in ethanol and soluble in solutions of alkali hydroxides or ammonia. PTU™ 50 mg Tablets are white, round, biconvex and uncoated. One side is debossed ‘PRESTAB’ and other side is plain. PHARMACOLOGY Category: Antithyroid Propylthiouracil blocks the peripheral conversion of thyroxine (T 4) to triiodothyronine (T 3) by inhibiting incorporation of iodide into tyrosine. Propylthiouracil is rapidly absorbed. The half-life in plasma approximates 2 hours (in anuric patients T½ 8.5 hours). Protein binding of propylthiouracil is approximately 75%. The drug is metabolized in the liver and is excreted in the bile (primary route) with approximately 30% being excreted in the urine as metabolites or whole drug. Propylthiouracil does not interfere with the action and the release of exogenous thyroid hormone. Clinical response, therefore, does not occur until circulating and colloid-stored thyroid hormone is utilised, and as such depends in part on the amount of colloid in the gland. The rapid fall in serum triiodothyronine T 3 concentration, before serum thyroxine (T 4) levels fall, parallels a clinical improvement in the thyrotoxic patient, and is generally seen after the first week. -

BASED NASAL FORMULATIONS of LEVOTHYROXINE by Esomchukwu
DEVELOPMENT AND IN VITRO CHARACTERIZATION OF POLYMER- BASED NASAL FORMULATIONS OF LEVOTHYROXINE by Esomchukwu Vin-Boris Obinna Submitted in partial fulfilment of the requirements for the degree of Masters of Science at Dalhousie University Halifax, Nova Scotia August 2020 © Copyright by Esomchukwu Vin-Boris Obinna, 2020 TABLE OF CONTENT LIST OF TABLES……………………………………………………………………… v LIST OF FIGURES……………………………………………………………………. vi ABSTRACT………………………………………………………………………………x LIST OF ABBREVIATIONS AND SYMBOLS USED………………………………xi ACKNOWLEDGEMENTS…….………………………………………………….… xii CHAPTER 1 INTRODUCTION ............................................................................. 1 1.1 ANATOMY AND PHYSIOLOGY OF THE THYROID GLAND .............................. 1 1.2 DISEASES OF THE THYROID GLAND ............................................................. 2 1.2.1 Hyperthyroidism ................................................................................. 2 1.2.2 Hypothyroidism .................................................................................. 3 1.3 LEVOTHYROXINE REPLACEMENT THERAPY ................................................. 4 1.3.1 Chemical and Physical Properties of Levothyroxine .......................... 5 1.3.2 Current Levothyroxine Delivery Methods .......................................... 6 1.4 ANATOMY AND PHYSIOLOGY OF THE NOSE ................................................. 8 1.5 NASAL DRUG DELIVERY .............................................................................. 9 1.5.1 Mechanisms of Nasal Drug Absorption -

Acting Via a Cell Surface Receptor, Thyroid Hormone Is a Growth Factor for Glioma Cells
Research Article Acting via a Cell Surface Receptor, Thyroid Hormone Is a Growth Factor for Glioma Cells Faith B. Davis,1 Heng-Yuan Tang,2 Ai Shih,1 Travis Keating,2 Lawrence Lansing,1 Aleck Hercbergs,5 Robert A. Fenstermaker,6 Ahmed Mousa,3 Shaker A. Mousa,3 Paul J. Davis,1,2,4 and Hung-Yun Lin1,2 1Ordway Research Institute, Inc.; 2Research Service, Stratton Veterans Affairs Medical Center; 3Albany College of Pharmacy; 4Wadsworth Center, New York State Department of Health, Albany, New York; 5Department of Radiation Oncology, The Cleveland Clinic, Cleveland, Ohio; and 6Department of Neurosurgery, Roswell Park Cancer Institute, Buffalo, New York Abstract RGD (arginine-glycine-aspartate) recognition site that is essential to the interactions of the integrin with its extracellular matrix Recent evidence suggests that the thyroid hormone L-thyroxine (ECM) protein ligands (2, 3). We have presented evidence that the (T4) stimulates growth of cancer cells via a plasma membrane thyroid hormone–binding site on integrin aVh3 is at or near the receptor on integrin AVB3.The contribution of this recently described receptor for thyroid hormone and receptor-based RGD recognition site and that an RGD peptide will displace stimulation of cellular mitogen-activated protein kinase thyroid hormone from integrin and will block the cellular actions [MAPK; extracellular signal-regulated kinase 1/2 (ERK1/2)] of the hormone (1). An interesting feature of this hormone activity, to enhancement of cell proliferation by thyroid receptor is that tetraiodothyroacetic acid, a hormone analogue hormone was quantitated functionally and by immunologic with reduced thyromimetic activity (4), competes with T4 at the means in three glioma cell lines exposed to T .At concen- receptor site (1). -

Pharm Endocrine Review
Pharm Endocrine Review Oral Hypoglycemic Medications α‐Glucosidase Insulin Secretagouges Insulin Sensitizer Inihibitors Sulfonylureas Meglitinides Phenylalanine Biguanides Thiazolidinediones Acarbose Miglitol Tolbutamide (1) Repaglinide Nateglinide Metformin Rosiglitazone Chloropramide (1) Pioglitazone Glyburide (2) Glipizide (2) Glimepiride (2) INSULIN SECRETAGOUGES The goal of these medications is simply to increase secretion of insulin from the pancreas. Each has its own nuances and proper usage, but essentially, they all regulate potassium efflux in the pancreatic β‐ islets causing an increased release of insulin in a still‐functioning pancreas Sulfonylureas. There are two generations of Sulfonylureas. The first generation (Tolbutamide and Chloropramide) are generally not used anymore because they have a risk of sulfur allergies. The second generation (Glyburide, Glipizide, Glimepiride) have no risk of sulfur allergies and are used much more frequently. Regardless of the generation, these function by blocking potassium efflux leading to depolarization of the pancreatic cells, calcium influx, and the release of insulin. Sulfonylureas can be taken just one time a day and are generally well tolerated. However, they run the risk of hypoglycemia, especially in those with hepatic failure. Meglitanides (Repaglinide). This functions just like a sulfonylurea: blocking potassium channels Æ increased insulin release. This differs from the sulfonylureas in that it has a rapid onset and short duration so is used as a supplement when eating. These are taken just prior to meals. When taken appropriately (i.e. you eat right after you take this) there is a lower risk of hypoglycemia. Of course, these drugs are new, which means expensive, and you must have a diligent diabetic who remembers to take his pill every time he eats. -

On Rana Pipiens Metamorphosis and Development
UNLV Theses, Dissertations, Professional Papers, and Capstones 2004 The Effects of environmentally relevant doses of Perchlorate (ClO4-) on Rana Pipiens metamorphosis and development Andrea N. Golli University of Nevada Las Vegas Follow this and additional works at: https://digitalscholarship.unlv.edu/thesesdissertations Part of the Desert Ecology Commons, Environmental Indicators and Impact Assessment Commons, and the Zoology Commons Repository Citation Golli, Andrea N., "The Effects of environmentally relevant doses of Perchlorate (ClO4-) on Rana Pipiens metamorphosis and development" (2004). UNLV Theses, Dissertations, Professional Papers, and Capstones. 183. http://dx.doi.org/10.34917/1436436 This Thesis is protected by copyright and/or related rights. It has been brought to you by Digital Scholarship@UNLV with permission from the rights-holder(s). You are free to use this Thesis in any way that is permitted by the copyright and related rights legislation that applies to your use. For other uses you need to obtain permission from the rights-holder(s) directly, unless additional rights are indicated by a Creative Commons license in the record and/ or on the work itself. This Thesis has been accepted for inclusion in UNLV Theses, Dissertations, Professional Papers, and Capstones by an authorized administrator of Digital Scholarship@UNLV. For more information, please contact [email protected]. THE EFFECTS OF ENVIRONMENTALLY RELEVANT DOSES - OF PERCHLORATE (ClO4 ) ON RANA PIPIENS METAMORPHOSIS AND DEVELOPMENT by Andrea N. Golli Bachelor of Science University of Findlay 2001 Master of Science University of Nevada, Las Vegas 2004 A thesis submitted in partial fulfillment of the requirements for the Master of Science Degree in Environmental Science Department of Environmental Studies Greenspun College of Urban Affairs Graduate College University of Nevada, Las Vegas May 2004 ABSTRACT The Effects of Environmentally Relevant Doses - of Perchlorate (ClO4 ) on Rana pipiens Metamorphosis and Development by Andrea N.