Bull-BMIG-30 Complete
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Subterranean Biodiversity and Depth Distribution of Myriapods in Forested Scree Slopes of Central Europe
A peer-reviewed open-access journal ZooKeys Subterranean930: 117–137 (2020) biodiversity and depth distribution of myriapods in forested scree slopes of... 117 doi: 10.3897/zookeys.930.48914 RESEARCH ARTICLE http://zookeys.pensoft.net Launched to accelerate biodiversity research Subterranean biodiversity and depth distribution of myriapods in forested scree slopes of Central Europe Beáta Haľková1, Ivan Hadrián Tuf 2, Karel Tajovský3, Andrej Mock1 1 Institute of Biology and Ecology, Faculty of Science, Pavol Jozef Šafárik University, Košice, Slovakia 2 De- partment of Ecology and Environmental Sciences, Faculty of Science, Palacky University, Olomouc, Czech Republic 3 Institute of Soil Biology, Biology Centre CAS, České Budějovice, Czech Republic Corresponding author: Beáta Haľková ([email protected]) Academic editor: L. Dányi | Received 28 November 2019 | Accepted 10 February 2020 | Published 28 April 2020 http://zoobank.org/84BEFD1B-D8FA-4B05-8481-C0735ADF2A3C Citation: Haľková B, Tuf IH, Tajovský K, Mock A (2020) Subterranean biodiversity and depth distribution of myriapods in forested scree slopes of Central Europe. In: Korsós Z, Dányi L (Eds) Proceedings of the 18th International Congress of Myriapodology, Budapest, Hungary. ZooKeys 930: 117–137. https://doi.org/10.3897/zookeys.930.48914 The paper is dedicated to Christian Juberthie (12 Mar 1931–7 Nov 2019), the author of the concept of MSS (milieu souterrain superficiel) and the doyen of modern biospeleology Abstract The shallow underground of rock debris is a unique animal refuge. Nevertheless, the research of this habitat lags far behind the study of caves and soil, due to technical and time-consuming demands. Data on Myriapoda in scree habitat from eleven localities in seven different geomorphological units of the Czech and Slovak Republics were processed. -

Wales: River Wye to the Great Orme, Including Anglesey
A MACRO REVIEW OF THE COASTLINE OF ENGLAND AND WALES Volume 7. Wales. River Wye to the Great Orme, including Anglesey J Welsby and J M Motyka Report SR 206 April 1989 Registered Office: Hydraulics Research Limited, Wallingford, Oxfordshire OX1 0 8BA. Telephone: 0491 35381. Telex: 848552 ABSTRACT This report reviews the coastline of south, west and northwest Wales. In it is a description of natural and man made processes which affect the behaviour of this part of the United Kingdom. It includes a summary of the coastal defences, areas of significant change and a number of aspects of beach development. There is also a brief chapter on winds, waves and tidal action, with extensive references being given in the Bibliography. This is the seventh report of a series being carried out for the Ministry of Agriculture, Fisheries and Food. For further information please contact Mr J M Motyka of the Coastal Processes Section, Maritime Engineering Department, Hydraulics Research Limited. Welsby J and Motyka J M. A Macro review of the coastline of England and Wales. Volume 7. River Wye to the Great Orme, including Anglesey. Hydraulics Research Ltd, Report SR 206, April 1989. CONTENTS Page 1 INTRODUCTION 2 EXECUTIVE SUMMARY 3 COASTAL GEOLOGY AND TOPOGRAPHY 3.1 Geological background 3.2 Coastal processes 4 WINDS, WAVES AND TIDAL CURRENTS 4.1 Wind and wave climate 4.2 Tides and tidal currents 5 REVIEW OF THE COASTAL DEFENCES 5.1 The South coast 5.1.1 The Wye to Lavernock Point 5.1.2 Lavernock Point to Porthcawl 5.1.3 Swansea Bay 5.1.4 Mumbles Head to Worms Head 5.1.5 Carmarthen Bay 5.1.6 St Govan's Head to Milford Haven 5.2 The West coast 5.2.1 Milford Haven to Skomer Island 5.2.2 St Bride's Bay 5.2.3 St David's Head to Aberdyfi 5.2.4 Aberdyfi to Aberdaron 5.2.5 Aberdaron to Menai Bridge 5.3 The Isle of Anglesey and Conwy Bay 5.3.1 The Menai Bridge to Carmel Head 5.3.2 Carmel Head to Puffin Island 5.3.3 Conwy Bay 6 ACKNOWLEDGEMENTS 7 REFERENCES BIBLIOGRAPHY FIGURES 1. -

Woodlice in Britain and Ireland: Distribution and Habitat Is out of Date Very Quickly, and That They Will Soon Be Writing the Second Edition
• • • • • • I att,AZ /• •• 21 - • '11 n4I3 - • v., -hi / NT I- r Arty 1 4' I, • • I • A • • • Printed in Great Britain by Lavenham Press NERC Copyright 1985 Published in 1985 by Institute of Terrestrial Ecology Administrative Headquarters Monks Wood Experimental Station Abbots Ripton HUNTINGDON PE17 2LS ISBN 0 904282 85 6 COVER ILLUSTRATIONS Top left: Armadillidium depressum Top right: Philoscia muscorum Bottom left: Androniscus dentiger Bottom right: Porcellio scaber (2 colour forms) The photographs are reproduced by kind permission of R E Jones/Frank Lane The Institute of Terrestrial Ecology (ITE) was established in 1973, from the former Nature Conservancy's research stations and staff, joined later by the Institute of Tree Biology and the Culture Centre of Algae and Protozoa. ITE contributes to, and draws upon, the collective knowledge of the 13 sister institutes which make up the Natural Environment Research Council, spanning all the environmental sciences. The Institute studies the factors determining the structure, composition and processes of land and freshwater systems, and of individual plant and animal species. It is developing a sounder scientific basis for predicting and modelling environmental trends arising from natural or man- made change. The results of this research are available to those responsible for the protection, management and wise use of our natural resources. One quarter of ITE's work is research commissioned by customers, such as the Department of Environment, the European Economic Community, the Nature Conservancy Council and the Overseas Development Administration. The remainder is fundamental research supported by NERC. ITE's expertise is widely used by international organizations in overseas projects and programmes of research. -

7 Vilisics F
ÁLLATTANI KÖZLEMÉNYEK (2010) 95(1) : 87–120. Újabb adatok Magyarország szárazföldi ászkarákfaunájához (Crustacea, Isopoda, Oniscidea) VILISICS FERENC és HORNUNG ERZSÉBET Szent István Egyetem, Állatorvos-tudományi Kar, Biológiai Intézet, Ökológiai Tanszék, H–1077 Budapest, Rottenbiller u. 50. E–mail: [email protected] Összefoglaló. Az elmúlt évtizedben Magyarországon számos ökológiai és faunisztikai vizsgálat tör- tént a szárazföldi ászkarákok (Isopoda : Oniscidea) csoportját érint ően. A gy űjtéseket azonban nem mindig követte az eredmények publikálása, holott az új adatok sok értékes kiegészít ő információt nyújtanak az egyes fajok hazai elterjedésér ől, valamint az egyes földrajzi tájegységek és él őhely- típusok ászkarák-együtteseinek összetételér ől. Adatbázisunkban 394, máig leközöletlen rekord talál- ható, amelyek a szerz ők módszeres gy űjtésének, valamint más talajzoológiai jelleg ű kutatásokból származó ászkarákanyag feldolgozásának eredményei. Ezen adatbázis összesen 48 ászkafaj eddig publikálatlan elterjedési adatát tartalmazza, ami a hazai fauna (57 faj) 84%-át teszi ki. Közülük figye- lemre méltó a hazánkból el őször 2005-ben leírt Trichoniscus steinboecki széles magyarországi elter- jedtségének igazolása. Korábban ritkának tartott (pl. Androniscus roseus, Armadillidium versicolor ), illetve csak üvegházinak ismert fajok ( Buddelundiella cataractae és Armadillidium nasatum ) szabad- földi el őfordulásait is itt közöljük, valamint egy hazánkban kihaltnak vélt faj ( Porcellio dilatatus ) el ő- fordulását is meger ősítjük. A mintavételi területek közül kiemelend ők az eddig alulreprezentált terüle- tekr ől [Kisalföld, Őrség, Alföld (Mez őföld, Bugac, Fels ő-Tiszavidék, Hortobágy)] származó adatok. Kulcsszavak : elterjedés, faunisztikai adatok, ritka fajok. Bevezetés és módszerek A szárazföldi ászkarákfajok hazai elterjedési adatait összefoglaló jegyzék (FORRÓ & FARKAS 1998) 42 ászkafajról közölt adatokat. Az ezt követ ő években sorra megjelen ő pub- likációkban (pl. -

Millipedes As Host for Microbes - a Review
International Journal of Microbiological Research 8 (1): 19-24, 2017 ISSN 2079-2093 © IDOSI Publications, 2017 DOI: 10.5829/idosi.ijmr.2017.19.24 Millipedes as Host for Microbes - A Review Anbarasan Dhivya and Periasamy Alagesan Post Graduate and Research Department of Zoology, Yadava College, Madurai, India Abstract: This review mainly highlights the microbial load that abode in the gut of different millipede species. These diplopods are capable of digesting the leaf litter which they feed was only by the aid of microbial communities which have the ability to produce various hydrolytic enzymes that are highly responsible for the degradation of feed consumed by the millipedes. Millipedes are not well equipped for the degradation of the plant detritus, thereby maintaining a symbiotic relationship with bacteria, makes millipedes as effective decomposers. Reports are also available on the existence of microbial populations in the soil, leaf litter and the faecal pellets of millipedes. The microbes lodging in the gut may expel along with faeces, so some species act as coprophagy, which feed on their own faecal material. Interestingly, the journey of leaf litter through the intestinal wall, results in repopulation of feces with bacteria while reaching the hindgut. This review makes clear, that litter alone may not be the feed of millipedes instead, microorganisms may be the targeted food source which hires the litter as a vehicle for consumption of microbes. Key words: Millipedes Gut microbes Decomposers Coprophagy INTRODUCTION the decomposition processes of decaying plant material. The food source of the millipedes is the forest Millipedes are terrestrial arthropods and third diverse litter. -

Some Aspects of the Ecology of Millipedes (Diplopoda) Thesis
Some Aspects of the Ecology of Millipedes (Diplopoda) Thesis Presented in Partial Fulfillment of the Requirements for the Degree Master of Science in the Graduate School of The Ohio State University By Monica A. Farfan, B.S. Graduate Program in Evolution, Ecology, and Organismal Biology The Ohio State University 2010 Thesis Committee: Hans Klompen, Advisor John W. Wenzel Andrew Michel Copyright by Monica A. Farfan 2010 Abstract The focus of this thesis is the ecology of invasive millipedes (Diplopoda) in the family Julidae. This particular group of millipedes are thought to be introduced into North America from Europe and are now widely found in many urban, anthropogenic habitats in the U.S. Why are these animals such effective colonizers and why do they seem to be mostly present in anthropogenic habitats? In a review of the literature addressing the role of millipedes in nutrient cycling, the interactions of millipedes and communities of fungi and bacteria are discussed. The presence of millipedes stimulates fungal growth while fungal hyphae and bacteria positively effect feeding intensity and nutrient assimilation efficiency in millipedes. Millipedes may also utilize enzymes from these organisms. In a continuation of the study of the ecology of the family Julidae, a comparative study was completed on mites associated with millipedes in the family Julidae in eastern North America and the United Kingdom. The goals of this study were: 1. To establish what mites are present on these millipedes in North America 2. To see if this fauna is the same as in Europe 3. To examine host association patterns looking specifically for host or habitat specificity. -

Ommatoiulus Moreleti (Lucas) and Cylindroiulus
Bulletin of the British Myriapod & Isopod Group Volume 30 (2018) OMMATOIULUS MORELETI (LUCAS) AND CYLINDROIULUS PYRENAICUS (BRÖLEMANN) NEW TO THE UK (DIPLOPODA, JULIDA: JULIDAE) AND A NEW HOST FOR RICKIA LABOULBENIOIDES (LABOULBENIALES) Steve J. Gregory1, Christian Owen2, Greg Jones and Emma Williams 1 4 Mount Pleasant Cottages, Church Street, East Hendred, Oxfordshire, OX12 8LA, UK. E-mail: [email protected] 2 75 Lewis Street, Aberbargoed. CF8 19DZ, UK. E-mail: [email protected] ABSTRACT The schizophylline millipede Ommatoiulus moreleti (Lucas) and the cylindroiuline millipede Cylindroiulus pyrenaicus (Brölemann) (Julida: Julidae) are recorded new for the UK from a site near Bridgend, Glamorganshire, in April 2017. An unidentified millipede first collected in April 2004 from Kenfig Burrows, Glamorganshire, is also confirmed as being C. pyrenaicus. Both species are described and illustrated, enabling identification. C. pyrenaicus is reported as a new host for the Laboulbeniales fungus Rickia laboulbenioides. Summary information is provided on habitat preferences of both species in South Wales and on their foreign distribution and habitats. It is considered likely that both species have been unintentionally introduced into the UK as a consequence of industrial activity in the Valleys of south Wales. INTRODUCTION The genera Ommatoiulus Latzel, 1884 and Cylindroiulus Verhoeff, 1894 (Julida: Julidae) both display high species diversity (Kime & Enghoff, 2017). Of the 47 described species of Ommatoiulus the majority are found in North Africa and the Iberian Peninsula (ibid). Currently, just one species, Ommatoiulus sabulosus (Linnaeus, 1758), is known from Britain and Ireland, a species that occurs widely across northern Europe (Kime, 1999) and in Britain reaches the northern Scottish coastline (Lee, 2006). -
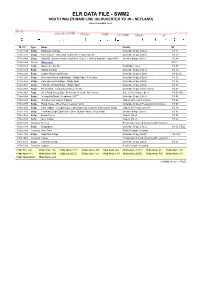
Elr Data File - Swm2 South Wales Main Line (Gloucester Yd Jn - Neyland)
ELR DATA FILE - SWM2 SOUTH WALES MAIN LINE (GLOUCESTER YD JN - NEYLAND) www.railwaydata.co.uk M. Ch. Type Name Details ID 113m 11ch Bridge Millstream Subway Underline Bridge | Steel 113 11 113m 13ch Bridge River Twyver - Also Under Chl At 92 78. Over Stream Underline Bridge | Steel 113 13 113m 44ch Bridge Windmill - Eastern Radial Road Over. Glos C.c. Own & Maintain - Agmt.rt510 Overline Bridge | Steel 113 44 114m 04ch Station Gloucester GCR 114m 07ch Bridge Gloucester Stn Fb - Footbridge | Steel 114 07 114m 12ch Bridge Station Subway Underline Bridge | Steel 114 12 114m 22ch Bridge London Road Underbridge Underline Bridge | Steel 114 21.25 114m 32ch Bridge Worcester Street Underbridge - Single Span. A38 Under Underline Bridge | Steel 114 32 114m 35ch Bridge Hare Lane Underbridge - Single Span Underline Bridge | Brick 114 35 114m 36ch Bridge Park Street Underbridge - Single Span Underline Bridge | Brick 114 36 114m 47ch Bridge Deans Walk - Closed To Vehicular Traffic Underline Bridge | Brick (Arch) 114 47 114m 54ch Bridge Over Road On Up Side. (fb Not On Nr Land - No Exams) Side of Line Bridge | Steel 114 54 B/S 114m 54ch Bridge "st.oswalds Road - Headroom 16'0""" Underline Bridge | Steel 114 54 115m 02ch Bridge St Catherines Viaduct 7 Spans Viaduct | Pre-cast Concrete 115 02 115m 07ch Bridge Pump House - River Severn (eastern Arm) Underline Bridge | Pre-tensioned Concrete 115 07 115m 16ch Bridge Ham Viaduct - 12 Spans (pre Cast Segmental Concrete Arches Over Land) Viaduct | Pre-cast Concrete 115 16 115m 31ch Bridge Townham Single Span -

Bridgend Sites of Importance for Nature Conservation Review December 2011
Bridgend Sites of Importance for Nature Conservation Review December 2011 Quality Management Job No GC/001170 Doc No. Title Sites of Importance for Nature Conservation Review Location Bridgend Document Ref File reference Date December 2011 Sarah Simons Prepared by Principal Ecologist 19-12-11 BSc (Hons), MSc, MIEEM Lucy Fay Checked by Senior Ecologist 19-12-11 BSc (Hons), AIEEM Ian Walsh Authorised by Technical Director 19-12-11 BSc, CEng, MICE, MCIHT Contents 1. INTRODUCTION 1 1.1 Background 1 1.2 Review objectives 1 2. METHODOLOGY 2 2.1 Introduction 2 2.2 Desktop Study 2 2.3 Field Survey 3 2.4 Constraints 4 3. OVERVIEW OF RESULTS 5 3.1 Sites that no longer qualify as SINCS 5 3.2 Sites that require re-survey to confirm status 6 3.3 Sites that should be upgraded from cSINC to SINC 6 4. RECOMMENDATIONS 7 4.1 Identifying new SINCs 7 4.2 SINC administration 7 4.3 SINC habitat management 8 5. REFERENCES 10 APPENDICES 11 i 1. INTRODUCTION 1.1 BACKGROUND Bridgend County Borough Council commissioned Capita Symonds to carry out a review of its Sites of Importance for Nature Conservation (SINCs). The review included both desk-based studies and field work. This report will provide a record of the methodologies used and a summary of the findings of the review along with recommendations for further work. 1.2 REVIEW OBJECTIVES • To confirm or otherwise that the Council’s suite of SINCs meet a robust set of criteria (Wildlife Sites Guidance Wales – A Guide to Develop Local Wildlife Systems in Wales (Wales Biodiversity Partnership, 2008)) • To provide SINC data sufficient to inform the emerging Local Development Plan (LDP) and the planning process. -
A Synopsis of Estonian Myriapod Fauna
A peer-reviewed open-access journal ZooKeys 793: 63–96 (2018) A synopsis of Estonian myriapod fauna... 63 doi: 10.3897/zookeys.793.28050 CHECKLIST http://zookeys.pensoft.net Launched to accelerate biodiversity research A synopsis of Estonian myriapod fauna (Myriapoda: Chilopoda, Diplopoda, Symphyla and Pauropoda) Kaarel Sammet1, Mari Ivask2, Olavi Kurina1 1 Institute of Agricultural and Environmental Sciences, Estonian University of Life Sciences, Kreutzwaldi st 5D, 51006 Tartu, Estonia 2 Tallinn University of Technology, Tartu College, Puiestee 78, 51008 Tartu, Estonia Corresponding author: Kaarel Sammet ([email protected]) Academic editor: Nesrine Akkari | Received 28 June 2018 | Accepted 17 September 2018 | Published 29 October 2018 http://zoobank.org/0D7B2412-5585-4AEA-81D4-1854A4415128 Citation: Sammet K, Ivask M, Kurina O (2018) A synopsis of Estonian myriapod fauna (Myriapoda: Chilopoda, Diplopoda, Symphyla and Pauropoda). ZooKeys 793: 63–96. https://doi.org/10.3897/zookeys.793.28050 Abstract The data on Estonian Myriapoda are scattered in various publications and there has been no overview of the fauna up to the present. A critical summary of the previous information on Estonian Myriapoda is given, supplemented by new records and distribution maps. Altogether, 5784 specimens from 276 col- lecting sites were studied. To the hitherto recorded 14 centipede species are added Lithobius melanops, L. microps, Geophilus carpophagus, G. flavus, Strigamia transsilvanica and Stenotaenia linearis, a probably introduced species. Of the 27 published Estonian millipede species, the data on two species proved er- roneous, and two new species were recorded (Craspedosoma raulinsii and Cylindroiulus britannicus). Two previously recorded millipede species – Brachyiulus pusillus and Mastigophorophyllon saxonicum – were not found in the recent samples, the latter may have become more rare or extinct. -

The Belgian Millipede Fauna (Diplopoda)
I I BULLETIN DE L'INSTITUT ROYAL DES SCIENCES NATURELLES DE BELGIQUE ENTOMOLOGIE, 74: 35-68, 200ll BULLETIN VAN HET KONINKLIJK BELGISCH INSTITUUT VOOR NATUURWETENSCHAPPEN ENTOMOLOGIE, 74: 35-68, 2004 The Belgian Millipede Fauna (Diplopoda) by R. Desmond KIME Summary (BIERNAUX, 1972) and the first Belgian millipede atlas concerning these two groups (BIERNAUX, 1971). Between The work done in the past on Belgian millipedes is briefly reviewed 1977 and 1980 MARQUET made a huge collection of soil and previous publications on the subject are cited. A check-list of Belgian Diplopoda is given. Biogeographical districts of Belgium are invertebrates taken from all regions of the country for the indicated and the distribution of millipedes within these is noted. Each Belgian Royal Institute ofNatural Science, the millipedes spedes is reviewed; its distribution in Belgium is related to its of which I identified and catalogued at the request of European geographical range and to knowledge of its ecology. Maps of distribution within Belgium are presented. There is discussion of the Dr Leon BAERT. My own collecting in Belgium began in origins, evolution and community composition of the Belgian milli 1974 and has continued to the present day. Detailed pede fauna. ecological studies were carried out from 1977 onwards, based in the laboratory of Professor Key words: Diplopoda, Belgium, check-list, biogeography, ecology, Philippe LEBRUN at faunal origins. Louvain-la-Neuve, some of these with colleagues at Gembloux, and at the Belgian Royal Institute of Natural Science. These studies gave rise to a number of publica tions adding to the knowledge of the Belgian fauna (KIME Introduction & WAUTHY, 1984; BRANQUART, 1991; KIME, 1992; KIME et al., 1992; BRANQUART et al., 1995, KIME, 1997). -

Notes on Authorship, Type Material and Current Systematic Position of the Diplopod Taxa Described by Hilda K. Brade-Birks and S. Graham Brade-Birks
Bulletin of the British Myriapod & Isopod Group Volume 25 (2011) NOTES ON AUTHORSHIP, TYPE MATERIAL AND CURRENT SYSTEMATIC POSITION OF THE DIPLOPOD TAXA DESCRIBED BY HILDA K. BRADE-BIRKS AND S. GRAHAM BRADE-BIRKS Graham S. Proudlove Department of Entomology, The Manchester Museum, The University of Manchester, Manchester M13 9PL. E-mail: [email protected] ABSTRACT H.K. Brade-Birks and S.G. Brade-Birks described eight Diplopoda taxa as new to science: two nominal genera and three nominal species in the Family Brachychaeteumatidae (Genera Jacksoneuma and Iacksoneuma, species Iacksoneuma bradeae, Brachychaeteuma melanops and B. quartum), one nominal species in the Family Blaniulidae (Proteroiulus pallidus), and two nominal subspecies in the Family Chordeumatidae (Chordeumella scutellare brolemanni and Chordeumella scutellare bagnalli). Over many years the fact that there were two Brade-Birks’ seems to have become forgotten and some of the authorships quoted for these names is certainly wrong because of this. This is discussed in detail and correct authorship for these names is provided. Similarly the nature and location of type material for these species is largely unknown and details of what is known are given where this has been ascertained. The currently accepted systematic position of these taxa is described. INTRODUCTION Hilda K. Brade (1890–1982) and S. Graham Birks (1887–1982) (who took the surname Brade-Birks on marriage, hereafter in the plural as Brade-Birks’) were the most important British myriapodologists of the early 20th century. In the period 1916 to 1939 they left us 36 of their “Notes on Myriapoda” which provided very valuable additions to knowledge of the British Diplopoda and Chilopoda, as well as synthesising the literature pertinent to British taxa.