P0148-P0159.Pdf
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Girl Scouts of Central Texas Explore Austin Patch Program
Girl Scouts of Central Texas Explore Austin Patch Program Created by the Cadette and Senior Girl Scout attendees of Zilker Day Camp 2003, Session 4. This patch program is a great program to be completed in conjunction with the new Capital Metro Patch Program available at gsctx.org/badges. PATCHES ARE AVAILABLE FOR PURCHASE IN GSCTX SHOPS. Program Grade Level Requirements: • Daisy - Ambassador: explore a minimum of eight (8) places. Email [email protected] if you find any hidden gems that should be on this list and share your adventures here: gsctx.org/share EXPLORE 1. Austin Nature and Science Center, 2389 Stratford Dr., (512) 974-3888 2. *The Contemporary Austin – Laguna Gloria, 700 Congress Ave. (512) 453-5312 3. Austin City Limits – KLRU at 26th and Guadalupe 4. *Barton Springs Pool (512) 867-3080 5. BATS – Under Congress Street Bridge, at dusk from March through October. 6. *Bob Bullock Texas State History Museum, 1800 Congress Ave. (512) 936-8746 7. Texas State Cemetery, 909 Navasota St. (512) 463-0605 8. *Deep Eddy Pool, 401 Deep Eddy. (512) 472-8546 9. Dinosaur Tracks at Zilker Botanical Gardens, 2220 Barton Springs Dr. (512) 477-8672 10. Elisabet Ney Museum, 304 E. 44th St. (512) 974-1625 11. *French Legation Museum, 802 San Marcos St. (512) 472-8180 12. Governor’s Mansion, 1010 Colorado St. (512) 463-5518 13. *Lady Bird Johnson Wildflower Center, 4801 La Crosse Ave. (512) 232-0100 14. LBJ Library 15. UT Campus 16. Mayfield Park, 3505 W. 35th St. (512) 974-6797 17. Moonlight Tower, W. 9th St. -

Free Land Attracted Many Colonists to Texas in 1840S 3-29-92 “No Quitting Sense” We Claim Is Typically Texas
“Between the Creeks” Gwen Pettit This is a compilation of weekly newspaper columns on local history written by Gwen Pettit during 1986-1992 for the Allen Leader and the Allen American in Allen, Texas. Most of these articles were initially written and published, then run again later with changes and additions made. I compiled these articles from the Allen American on microfilm at the Allen Public Library and from the Allen Leader newspapers provided by Mike Williams. Then, I typed them into the computer and indexed them in 2006-07. Lois Curtis and then Rick Mann, Managing Editor of the Allen American gave permission for them to be reprinted on April 30, 2007, [email protected]. Please, contact me to obtain a free copy on a CD. I have given a copy of this to the Allen Public Library, the Harrington Library in Plano, the McKinney Library, the Allen Independent School District and the Lovejoy School District. Tom Keener of the Allen Heritage Guild has better copies of all these photographs and is currently working on an Allen history book. Keener offices at the Allen Public Library. Gwen was a longtime Allen resident with an avid interest in this area’s history. Some of her sources were: Pioneering in North Texas by Capt. Roy and Helen Hall, The History of Collin County by Stambaugh & Stambaugh, The Brown Papers by George Pearis Brown, The Peters Colony of Texas by Seymour V. Conner, Collin County census & tax records and verbal history from local long-time residents of the county. She does not document all of her sources. -

San Jacinto Largest Floor Plates in Austin Cbd Under Construction
SAN1836 JACINTO 230,609 RSF DELIVERING Q1 2021 1836 SAN JACINTO LARGEST FLOOR PLATES IN AUSTIN CBD UNDER CONSTRUCTION Uniquely located within the Capitol Complex, adjacent to the bustling Innovation and Medical Districts. 1836 SAN JACINTO CONTENTS CLICK ICON TO NAVIGATE AN AUTHENTIC AUSTIN EXPERIENCE WITH ABUNDANT ON-SITE AND WALKABLE AMENITIES LOCATION REGIONAL AERIAL VIEW MOBILITY INTERSECTION OF DELL MEDICAL, AREA DEVELOPMENT UT, CAPITOL COMPLEX & INNOVATION DISTRICT CAPITOL COMPLEX AMENITIES ARTS & ENTERTAINMENT LARGEST FLOORPLATES WITHIN THE CBD FOOD & DRINKS OFFERING UNPARALLELED VIEWS GREENSPACE HOSPITALITY 1836 SAN JACINTO OFF-SET CORE DESIGN OVERVIEW DELIVERING EFFICIENT INTERIOR SPACE PROGRAMMING FEATURES GROUND FLOOR PLAN TYPICAL FLOOR PLAN TERRACE FLOOR PLAN EXTENSIVE 9TH FLOOR CONFERENCE CENTER & EVENT SPACE CONTACT FEATURING AN OUTDOOR TERRACE INFORMATION © April 25, 2019. CBRE. All Rights Reserved. INTERSECTION 1 OF INNOVATION Pflugerville 1836 SAN JACINTO UNIVERSITY OF TEXAS Lake Travis W 17 MLK The Jr. 16 JUDGE’S Blvd En The f Domain ie HILL ld Rd W Arboretum 17th 18361836 SAN SAN JACINTOJACINTO St d Lake Travis Jr. Blv E MLK W d 1 v 5th l St B o t n i UPTOWN c MEDICAL t a S J n d n n v a l CAPITOL y S L B 20 r COMPLEX 360 W W 1 ma 2th a St 16 L Bridge o SH-130 i N n o t CENTRAL & Hwy 290 n W WATERLOO HEALTH A 10th PARK West Lake 9 n St th St a E 12 Highland S t S WEST a c W a 5th END v St W CATALYST TO EMERGING DISTRICT 6th La St W AUSTIN, TX 8 7th Mueller St CONGRESS Ideally situated between the University MARKET INNOVATION e v A E W t 1 5 s 1 of Texas, Dell Medical School and Tex- Innovation th S S th S t y t es t r i in District ng r as Capitol, 1836 San Jacinto is well po- WAREHOUSE o T W C SEAHOLM 3r d St Downtown E sitioned to be the employment catalyst W C 7th Austin esa CONVENTION St r Cha Austin ve within Austin’s Innovation District. -

Mexican American History Resources at the Briscoe Center for American History: a Bibliography
Mexican American History Resources at the Briscoe Center for American History: A Bibliography The Briscoe Center for American History at the University of Texas at Austin offers a wide variety of material for the study of Mexican American life, history, and culture in Texas. As with all ethnic groups, the study of Mexican Americans in Texas can be approached from many perspectives through the use of books, photographs, music, dissertations and theses, newspapers, the personal papers of individuals, and business and governmental records. This bibliography will familiarize researchers with many of the resources relating to Mexican Americans in Texas available at the Center for American History. For complete coverage in this area, the researcher should also consult the holdings of the Benson Latin American Collection, adjacent to the Center for American History. Compiled by John Wheat, 2001 Updated: 2010 2 Contents: General Works: p. 3 Spanish and Mexican Eras: p. 11 Republic and State of Texas (19th century): p. 32 Texas since 1900: p. 38 Biography / Autobiography: p. 47 Community and Regional History: p. 56 The Border: p. 71 Education: p. 83 Business, Professions, and Labor: p. 91 Politics, Suffrage, and Civil Rights: p. 112 Race Relations and Cultural Identity: p. 124 Immigration and Illegal Aliens: p. 133 Women’s History: p. 138 Folklore and Religion: p. 148 Juvenile Literature: p. 160 Music, Art, and Literature: p. 162 Language: p. 176 Spanish-language Newspapers: p. 180 Archives and Manuscripts: p. 182 Music and Sound Archives: p. 188 Photographic Archives: p. 190 Prints and Photographs Collection (PPC): p. 190 Indexes: p. -

Strategic Plan Updated: September 2018
Harry Ransom Center Strategic Plan Updated: September 2018 TABLE OF CONTENTS Vision, Mission, and Values Page 1 From The Director Page 3 Goals Page 7 Goals, Strategies, and Action Steps Goal 1: Collections Page 8 Goal 2: Research, Scholarship, and Teaching Page 10 Goal 3: Public Engagement Page 12 Goal 4: Organizational Culture Page 15 Goal 5: Facility and Infrastructure Page 17 Goal 6: Resources Page 19 Acknowledgements Page 21 VISION The Ransom Center strives to be the leading research library and museum for the study and greater understanding of the literature and culture shaping our time. MISSION The Ransom Center encourages discovery, inspires creativity, and advances understanding of the humanities for a broad and diverse audience through the preservation and sharing of its extraordinary collections. ABOUT THE RANSOM CENTER The Ransom Center is an internationally renowned humanities research library and museum at The University of Texas at Austin. Its extensive collections provide unique insight into the creative process of writers and artists, deepening our understanding and appreciation of literature, photography, film, art, and the performing arts. Visitors engage with the Center’s collections through research and study, exhibitions, publications, and a rich variety of program offerings including readings, talks, symposia, and film screenings. VALUES Collection Development and Stewardship The Ransom Center is committed to building collections of enduring cultural value and caring for them in accordance with the highest standards of preservation and access. Public Service and Engagement The Ransom Center aspires to engage the broadest possible audience with its diverse and internationally renowned collections. Ransom Center Strategic Plan: Updated September 2018 1 Creativity and Innovation The Ransom Center values creativity and innovation in the materials we preserve, in the interpretation of these materials, and in our service to the public. -

TEXAS HERITAGE TRAIL Boy Scouts of America
Capitol Area Council TEXAS HERITAGE TRAIL Boy Scouts of America TRAIL REQUIREMENTS: 1. There should be at least one adult for each 10 hikers. A group must have an adult leader at all times on the trail. The Boy Scouts of America policy requires two adult leaders on all Scout trips and tours. 2. Groups should stay together while on the hike. (Large groups may be divided into several groups.) 3. Upon completion of the trail the group leader should send an Application for Trail Awards with the required fee for each hiker to the Capitol Area Council Center. (Only one patch for each participant.) The awards will be mailed or furnished as requested by the group leader. Note: All of Part One must be hiked and all points (1-15) must be visited. Part Two is optional. HIKER REQUIREMENTS: 1. Any registered member of the Boy Scouts of America, Girl Scouts, or other civic youth group may hike the trail. 2. Meet all Trail requirements while on the hike. 3. The correct Scout uniform should be worn while on the trail. Some article (T-shirt, armband, etc) should identify other groups. 4. Each hiker must visit the historical sites, participate in all of his/her group’s activities, and answer the “On the Trail Quiz” to the satisfaction of his/her leader. Other places of interest you may wish to visit are: Zilker Park and Barton Springs Barton Springs Road Elisabet Ney Museum 304 E. 34th. Street Hike and Bike Trail along Town Lake Camp Mabry 38th. Street Lake Travis FM #620 Lake Austin FM # 2222 Capitol Area Council TEXAS HERITAGE TRAIL Boy Scouts of America ACCOMODATIONS: McKinney Falls State Park, 5805 McKinney Falls Parkway, Austin, TX 78744, tel. -
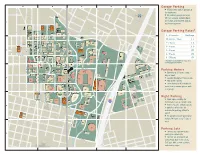
Parking Map for UT Campus
Garage Parking n Visitors may park in garages at the hourly rate n All parking garages are open 24/7 on a space-available basis for visitors and students and do not require a permit Garage Parking Rates* 0-30 minutes No Charge 30 minutes - 1 hour $ 3 1 - 2 hours $ 6 2 - 3 hours $ 9 3 - 4 hours $12 4 - 8 hours $15 8 - 24 hours $18 * Rates and availability may vary during special events. Parking Meters n Operational 24 hours a day, 7 days a week n Located throughout the campus n 25¢ for 15 minutes n Time limited to 45 minutes. If more time is needed, please park in a garage Night Parking n Read signs carefully for restrictions such as “At All Times” Bob B n ulloc After 5:45 p.m., certain spaces Texas k State Histo M ry useum in specific surface lots are available for parking without a permit n All garages provide parking for visitors 24 hours a day, 7 days a week Parking Lots n There is no daytime visitor parking in surface lots n Permits are required in all Tex surface lots from 7:30 a.m. to as Sta Ca te pitol 5:45 p.m. M-F as well as times indicated by signs BUILDING DIRECTORY CRD Carothers Dormitory .............................A2 CRH Creekside Residence Hall ....................C2 J R Public Parking CS3 Chilling Station No. 3 ...........................C4 JCD Jester Dormitory ..................................... B4 RHD Roberts Hall Dormitory .........................C3 CS4 Chilling Station No. 4 ...........................C2 BRG Brazos Garage .....................................B4 JES Beauford H. Jester Center ....................B3 RLM Robert Lee Moore Hall ..........................B2 CS5 Chilling Station No. -

Faculty & Staff Parking
FACULTY & STAFF PARKING PARKING & TRANSPORTATION SERVICES | 15/16 FACULTY & STAFF PARKING to notify all permit holders of special CONTACT CONTENTS events that affect parking (see Special PTS MAIN OFFICE 02 Contact Events Calendar on PTS website). 1815 TRINITY ST. 02 Parking at UT Office Hours: FACULTY & STAFF 03 Permit/Parking Options M-F | 8 am–5 pm WITH DISABILITIES 04 Building Index Cashier Hours: PTS offers both annual “D” and temporary M-F | 8 am–7 pm 05 Campus Map “TD” permits for faculty/staff with permanent and temporary disabilities. Phone: 512-471-PARK (7275) 06 Citations, Booting & Appeals To obtain a “D” permit, faculty/staff must Fax: 512-232-9405 06 Driving & Parking Offenses bring a copy of their state ADA placard Mail: PO Box, Austin, TX 78713- 07 Green on the Go to any staffed garage office. If only an ADA license plate is available, the “D” 7546, Campus Mail D3000 08 Parking 101 permit must be purchased Monday Online: www.utexas.edu/parking through Friday from 8 a.m. to 5 p.m. If Twitter: utaustinparking PARKING AT UT the disability is temporary in nature , “TD” Located in the center of the city, The permits are issued at a rate of $12/month University of Texas at Austin campus is upon receipt of a letter or fax from the GARAGES often congested, making commuting applicant’s doctor stating the nature and Brazos Garage and parking difficult. Understanding duration of the temporary disability. 512-471-6126 (BRG) your parking and transportation options Alternative parking is provided for Conference Center and regulations will make campus “D” permit holders unable to locate 512-232-8314 Garage (CCG) access easier and promote safety. -

Schizomus Siamensis (Schizomida: Schizomidae) from Eastern Asia and Hawaii
ACTA ARACHNOL., 35: 23-28, 1986 23 SCHIZOMUS SIAMENSIS (SCHIZOMIDA: SCHIZOMIDAE) FROM EASTERN ASIA AND HAWAII James C. COKENDOLPHER Department of Entomology, Texas Tech University, Lubbock, Texas 79409, U. S. A. and James R. REDDELL Texas Memorial Museum, The University of Texas at Austin, 2400 Trinity, Austin, Texas 78705, U. S. A. Synopsis COKENDOLPHER,James C. (Department of Entomology, Texas Tech University, Lubbock, Texas 79409, U. S. A.) and James R. REDDELL(Texas Memorial Museum, The University of Texas at Austin, 2400 Trinity, Austin, Texas 78705, U. S. A.) Schizomus siamensis (Schizomida : Schizomidae) from eastern Asia and Hawaii. Acta arachnol., 35: 23-28 (1986). A male lectotype and paralectotypes (male and female) are designated from material collected in Thailand. A complete synonymy and records from Hong Kong, Ryukyu Islands of Japan, and Hawaiian Islands of U. S. A, are provided. The female genitalia is illustrated for the first time. Schizomids from eastern Asia have been transferred back and forth from Schizomus COOK to Trithyreus KRAEPELIN without knowledge of the type species of either genus [see SEKIGUCHI and YAMASAKI (1975) and REDDELL and COK- ENDOLPHER (1985) for a review of the problem.] We have studied type speci- mens of these species and find that both genera are distinct, but that they can not be separated on the baisis of the metapeltidium (split or entire) (REDDELL and COKENDOLPHER,1985, and unpubi. data). Authors of recent papers on Schizomus siamensis have incorrectly placed this species in Trithyreus. It is the purpose of this contribution to record as complete as possible all collection locali- 24 J. -
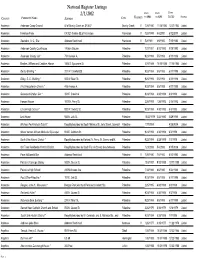
National Register Listings 2/1/2012 DATE DATE DATE to SBR to NPS LISTED STATUS COUNTY PROPERTY NAME ADDRESS CITY VICINITY
National Register Listings 2/1/2012 DATE DATE DATE TO SBR TO NPS LISTED STATUS COUNTY PROPERTY NAME ADDRESS CITY VICINITY AndersonAnderson Camp Ground W of Brushy Creek on SR 837 Brushy Creek V7/25/1980 11/18/1982 12/27/1982 Listed AndersonFreeman Farm CR 323 3 miles SE of Frankston Frankston V7/24/1999 5/4/2000 6/12/2000 Listed AndersonSaunders, A. C., Site Address Restricted Frankston V5/2/1981 6/9/1982 7/15/1982 Listed AndersonAnderson County Courthouse 1 Public Square Palestine7/27/1991 8/12/1992 9/28/1992 Listed AndersonAnderson County Jail * 704 Avenue A. Palestine9/23/1994 5/5/1998 6/11/1998 Listed AndersonBroyles, William and Caroline, House 1305 S. Sycamore St. Palestine5/21/1988 10/10/1988 11/10/1988 Listed AndersonDenby Building * 201 W. Crawford St. Palestine9/23/1994 5/5/1998 6/11/1998 Listed AndersonDilley, G. E., Building * 503 W. Main St. Palestine9/23/1994 5/5/1998 6/11/1998 Listed AndersonFirst Presbyterian Church * 406 Avenue A Palestine9/23/1994 5/5/1998 6/11/1998 Listed AndersonGatewood-Shelton Gin * 304 E. Crawford Palestine9/23/1994 4/30/1998 6/3/1998 Listed AndersonHoward House 1011 N. Perry St. Palestine3/28/1992 1/26/1993 3/14/1993 Listed AndersonLincoln High School * 920 W. Swantz St. Palestine9/23/1994 4/30/1998 6/3/1998 Listed AndersonLink House 925 N. Link St. Palestine10/23/1979 3/24/1980 5/29/1980 Listed AndersonMichaux Park Historic District * Roughly bounded by South Michaux St., Jolly Street, Crockett Palestine1/17/2004 4/28/2004 Listed AndersonMount Vernon African Methodist Episcopal 913 E. -

Bibliography of Sources
Texas Navy Association Historical Article Bibliography Basic Histories of the Texas Navy Borden, G. & T. H., Rules, Regulations, and Instructions for the Douglas, Claude L, Thunder on the Gulf, or, The Story of the Texas Naval Service of the Republic of Texas, Columbia, 1837 Navy, Turner Company, 1936; reprint, Old Army Press, 1973, 128p. Cussler, Clive, The Sea Hunters, P.63-75, Simon & Schuster, New York, New York, Eller, E. M., Introduction, The Texas Navy, Naval History Division, 1996, 364p. Department of the Navy, Washington, D.C., 1968, 40p. Dawson, Frederick, Petition of Frederick Dawson, James Schott, and Elisha Fischer, Ernest G., Robert Potter, Founder of the Texas Navy, Peli- Dana Whitney, Praying Payment for Certain Vessels, Etc., can Publishing Furnished Texas and Company, Gretna, Louisiana, 1976, 258p. Given Up by Texas to the U.S. on the Annexation of Texas, Senate, 30th Congress, Francaviglia, Richard V., From Sail to Steam, Four Centuries of first session, Misc. No. 27, Washington Government Printing Texas Maritime Office, 1848, 6p. History 1500-1900, University of Texas Press, Austin, Texas, 1998, 324p. Devereaux, Linda Ericson, The Texas Navy, Ericson Books, Nacogdoches, Texas, 1983 Guthrie, Keith, Texas Forgotten Ports, 3 volumes, Carnegie Insti- tution, Dienst, Dr. Alexander, The Navy of the Republic of Texas, 1835- Washington, D.C., 1923, 1926, vol. 3, Eaton Press, Austin, 1845 , privately Texas, 1993, 291p. published, Temple, Texas, Quarterly of the Texas State His- torical Association Hill, Jim Dan, The Texas Navy, in Forgotten Battles and Shirt- (January-April 1909), 165-203p.; reprint, Old Army Press, sleeve Diplomacy, Fort Collins, University of Chicago Press, 1937; reprint, State House Press, Colorado, 1987 Austin, Texas, 1987, 224p. -

Girlday.Utexas.Edu @UTWEP / #Girlday2018
girlday.utexas.edu @UTWEP / #GirlDay2018 Download the app at girlday.utexas.edu/app Girl Day at UT Austin Day irl G at UT Austin saturday, february 24 · 2018 INTRODUCE A GIRL TO ENGINEERING DAY schedule presented by 11:30 am - 5 pm activities, demos, shows details inside “Future Technologies” Stage presented by BAE Systems 11:30 AM WELCOME with Halliburton 12 PM Welcome with BAE Systems 12, 1:15 & 2:30 pm UT Austin physics circus · 1 hour shows 4 PM science in the movies stem stunt show · 1 hour show girl day stem festival presented by 5 PM introduce a girl to engineering day closing “Creating Chemistry” Stage presented by BASF Corporation 12 PM WElcome with BASF Corporation 12:30 & 1:30 PM UT Austin frozen chemistry · 30 minute shows 3 & 4 pm ut austin fun with chemistry · 30 minute shows 4:30 pm Girl Day STEM Festival Closing with Texas Instruments About the women in engineering program Connecting students, educators & professionals to the world of engineering through recruitment, education & support initiatives to promote the success & advancement of women in engineering. engr.utexas.edu/wep Introduce a Girl to Engineering Day presented by K - 3rd Grade CPE BUILDING HOST: INTEL CPE 2.202 ................................... Simple Concepts in Subsurface Engineering | UT Austin Department of Petroleum and Geosystems Engineering CPE 2.204 .................................. Balloon Car Race Challenge | Intel CPE 2.206 .................................. Bunny Copters - Paper Helicopters | Girl Scouts of Central Texas, American Society of Heating, Refrigeration, and Air Conditioning CPE 2.208 .................................. Bubble Shapes: Create a Bubble Wand | UT Austin Student Engineering Council CPE 2.210 ..................................