VIEW Open Access Recent Advances in the Treatment of Skin Involvement in Systemic Sclerosis Yoshihide Asano
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Fig. L COMPOSITIONS and METHODS to INHIBIT STEM CELL and PROGENITOR CELL BINDING to LYMPHOID TISSUE and for REGENERATING GERMINAL CENTERS in LYMPHATIC TISSUES
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date Χ 23 February 2012 (23.02.2012) WO 2U12/U24519ft ft A2 (51) International Patent Classification: AO, AT, AU, AZ, BA, BB, BG, BH, BR, BW, BY, BZ, A61K 31/00 (2006.01) CA, CH, CL, CN, CO, CR, CU, CZ, DE, DK, DM, DO, DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, (21) International Application Number: HN, HR, HU, ID, IL, IN, IS, JP, KE, KG, KM, KN, KP, PCT/US201 1/048297 KR, KZ, LA, LC, LK, LR, LS, LT, LU, LY, MA, MD, (22) International Filing Date: ME, MG, MK, MN, MW, MX, MY, MZ, NA, NG, NI, 18 August 201 1 (18.08.201 1) NO, NZ, OM, PE, PG, PH, PL, PT, QA, RO, RS, RU, SC, SD, SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, TM, (25) Filing Language: English TN, TR, TT, TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, (26) Publication Language: English ZW. (30) Priority Data: (84) Designated States (unless otherwise indicated, for every 61/374,943 18 August 2010 (18.08.2010) US kind of regional protection available): ARIPO (BW, GH, 61/441,485 10 February 201 1 (10.02.201 1) US GM, KE, LR, LS, MW, MZ, NA, SD, SL, SZ, TZ, UG, 61/449,372 4 March 201 1 (04.03.201 1) US ZM, ZW), Eurasian (AM, AZ, BY, KG, KZ, MD, RU, TJ, TM), European (AL, AT, BE, BG, CH, CY, CZ, DE, DK, (72) Inventor; and EE, ES, FI, FR, GB, GR, HR, HU, IE, IS, ΓΓ, LT, LU, (71) Applicant : DEISHER, Theresa [US/US]; 1420 Fifth LV, MC, MK, MT, NL, NO, PL, PT, RO, RS, SE, SI, SK, Avenue, Seattle, WA 98101 (US). -

Medical Therapy of Stricturing Crohn's Disease
Bettenworth and Rieder Fibrogenesis & Tissue Repair 2014, 7:5 http://www.fibrogenesis.com/content/7/1/5 REVIEW Open Access Medical therapy of stricturing Crohn’s disease: what the gut can learn from other organs - asystematicreview Dominik Bettenworth1† and Florian Rieder2,3*† Abstract Crohn’s disease (CD) is a chronic remitting and relapsing disease. Fibrostenosing complications such as intestinal strictures, stenosis and ultimately obstruction are some of its most common long-term complications. Despite recent advances in the pathophysiological understanding of CD and a significant improvement of anti-inflammatory therapeutics, medical therapy for stricturing CD is still inadequate. No specific anti-fibrotic therapy exists and the incidence rate of strictures has essentially remained unchanged. Therefore, the current therapy of established fibrotic strictures comprises mainly endoscopic dilation as well as surgical approaches. However, these treatment options are associated with major complications as well as high recurrence rates. Thus, a specific anti-fibrotic therapy for CD is urgently needed. Importantly, there is now a growing body of evidence for prevention as well as effective medical treatment of fibrotic diseases of other organs such as the skin, lung, kidney and liver. In face of the similarity of molecular mechanisms of fibrogenesis across these organs, translation of therapeutic approaches from other fibrotic diseases to the intestine appears to be a promising treatment strategy. In particular transforming growth factor beta (TGF-β) neutralization, selective tyrosine kinase inhibitors, blockade of components of the renin-angiotensin system, IL-13 inhibitors and mammalian target of rapamycin (mTOR) inhibitors have emerged as potential drug candidates for anti-fibrotic therapy and may retard progression or even reverse established intestinal fibrosis. -

In Vivo Imaging of Tgfβ Signalling Components Using Positron
REVIEWS Drug Discovery Today Volume 24, Number 12 December 2019 Reviews KEYNOTE REVIEW In vivo imaging of TGFb signalling components using positron emission tomography 1 1 2 Lonneke Rotteveel Lonneke Rotteveel , Alex J. Poot , Harm Jan Bogaard , received her MSc in drug 3 1 discovery and safety at the Peter ten Dijke , Adriaan A. Lammertsma and VU University in 1 Amsterdam. She is Albert D. Windhorst currently finishing her PhD at the VU University 1 Department of Radiology and Nuclear Medicine, Amsterdam UMC, location VUmc, Amsterdam, The Netherlands Medical Center (VUmc) 2 under the supervision of A. Pulmonary Medicine, Institute for Cardiovascular Research, Amsterdam UMC, location VUmc, Amsterdam, The Netherlands D. Windhorst and Adriaan A. Lammertsma. Her 3 research interest is on the development of positron Department of Cell and Chemical Biology, Oncode Institute, Leiden University Medical Center, Leiden, The emission tomography (PET) tracers that target Netherlands selectively the activin receptor-like kinase 5 in vitro and in vivo. Alex J. Poot obtained his The transforming growth factor b (TGFb) family of cytokines achieves PhD in medicinal chemistry homeostasis through a careful balance and crosstalk with complex from Utrecht University. As postdoctoral researcher at signalling pathways. Inappropriate activation or inhibition of this pathway the VUmc, Amsterdam, he and mutations in its components are related to diseases such as cancer, developed radiolabelled anticancer drugs for PET vascular diseases, and developmental disorders. Quantitative imaging of imaging. In 2014, he accepted a research expression levels of key regulators within this pathway using positron fellowship from Memorial Sloan Kettering Cancer 13 emission tomography (PET) can provide insights into the role of this Center, New York to develop C-labelled probes for tumour metabolism imaging with magnetic resonance in vivo pathway , providing information on underlying pathophysiological imaging (MRI). -

Biological Therapy in Systemic Sclerosis
Chapter 7 Biological Therapy in Systemic Sclerosis Joana Caetano, Susana Oliveira and JoanaJosé Delgado Caetano, Alves Susana Oliveira and AdditionalJosé Delgado information Alves is available at the end of the chapter Additional information is available at the end of the chapter http://dx.doi.org/10.5772/intechopen.69326 Abstract Systemic sclerosis is the autoimmune connective tissue disease with the highest morbid- ity and mortality, through the combination of inflammation, vasculopathy and fibrosis leading to severe internal organ involvement. Currently, there are no approved disease- modifying therapies, and treatment is based on organ-specific treatment and broad immu- nosuppression, with disappointing long-term results in most cases. Recent research has helped to improve knowledge of the pathogenesis of systemic sclerosis and to optimize treatment based on specific physiopathological targets, and a new era of biological agents in systemic sclerosis has now begun. Promising results are emerging from targeting spe- cific cytokine signalling, especially IL-6, and cellular subpopulations such as B cells, with anti-CD20 therapy, and T-cells, with inhibition of T-cell co-stimulation. Other approaches under evaluation are based on the modulation of profibrotic pathways by anti-TGF-β agents. In this chapter, we discuss the available evidence to support the use of each biologi- cal agent in systemic sclerosis based on data from basic and translational research and on results from clinical studies. Keywords: systemic sclerosis, biological therapy, cytokines, immune dysfunction, targeted treatment 1. Introduction Systemic sclerosis (SSc) is a rare multisystem connective tissue disease in which inflamma- tion, fibrosis, vasculopathy and autoimmunity are the principal features leading to diverse organ-based injuries [1]. -

Modifications to the Harmonized Tariff Schedule of the United States To
U.S. International Trade Commission COMMISSIONERS Shara L. Aranoff, Chairman Daniel R. Pearson, Vice Chairman Deanna Tanner Okun Charlotte R. Lane Irving A. Williamson Dean A. Pinkert Address all communications to Secretary to the Commission United States International Trade Commission Washington, DC 20436 U.S. International Trade Commission Washington, DC 20436 www.usitc.gov Modifications to the Harmonized Tariff Schedule of the United States to Implement the Dominican Republic- Central America-United States Free Trade Agreement With Respect to Costa Rica Publication 4038 December 2008 (This page is intentionally blank) Pursuant to the letter of request from the United States Trade Representative of December 18, 2008, set forth in the Appendix hereto, and pursuant to section 1207(a) of the Omnibus Trade and Competitiveness Act, the Commission is publishing the following modifications to the Harmonized Tariff Schedule of the United States (HTS) to implement the Dominican Republic- Central America-United States Free Trade Agreement, as approved in the Dominican Republic-Central America- United States Free Trade Agreement Implementation Act, with respect to Costa Rica. (This page is intentionally blank) Annex I Effective with respect to goods that are entered, or withdrawn from warehouse for consumption, on or after January 1, 2009, the Harmonized Tariff Schedule of the United States (HTS) is modified as provided herein, with bracketed matter included to assist in the understanding of proclaimed modifications. The following supersedes matter now in the HTS. (1). General note 4 is modified as follows: (a). by deleting from subdivision (a) the following country from the enumeration of independent beneficiary developing countries: Costa Rica (b). -

Treatment of Diffuse Cutaneous Systemic Sclerosis with Hyperimmune Caprine Serum
University College London (UCL) MD (Res) Degree Treatment of Diffuse Cutaneous Systemic Sclerosis with Hyperimmune Caprine Serum DR. NIAMH PATRICIA QUILLINAN 1 Declaration I, Niamh Patricia Quillinan, confirm that the work presented in this thesis is my own. Where information has been derived from other sources, I confirm that this has been indicated in the thesis. Signed: ___________________________________ Date: 17/03/2016 2 Abstract Systemic sclerosis (SSc) is a multisystem autoimmune rheumatic disorder with high morbidity and the highest case specific mortality of the rheumatic diseases. There is no currently approved unequivocally effective treatment for SSc and therefore there is a huge unmet medical need for novel and effective therapies. Hyperimmune caprine serum (HCS) is a goat serum extract derivative produced from goats vaccinated with a detergent-inactivated HIV viral lysate. It contains caprine immunoglobulins and small molecular weight proteins as well as a CRH, α-2 macroglobulin (α-2M) and lipoprotein-related peptide-1 complex. In this thesis we explore the hypothesis that hyperimmune caprine serum improves skin and other measures of disease severity in established dcSSc by modulating immunological function that determines persistence of clinical disease. This hypothesis is explored through 1) a prospective clinical trial, 2) long-term clinical use and 3) detailed assessment of serum growth factors and cytokines, as well as established and exploratory markers of disease. The primary objective of the clinical trial was to explore safety and tolerability of HCS in established diffuse cutaneous systemic sclerosis (dcSSc). Secondary objectives included assessment of potential efficacy and biological activity and exploration of candidate biomarkers. There were no safety concerns and frequency of adverse events was not different between HCS and placebo group. -

The Two Tontti Tudiul Lui Hi Ha Unit
THETWO TONTTI USTUDIUL 20170267753A1 LUI HI HA UNIT ( 19) United States (12 ) Patent Application Publication (10 ) Pub. No. : US 2017 /0267753 A1 Ehrenpreis (43 ) Pub . Date : Sep . 21 , 2017 ( 54 ) COMBINATION THERAPY FOR (52 ) U .S . CI. CO - ADMINISTRATION OF MONOCLONAL CPC .. .. CO7K 16 / 241 ( 2013 .01 ) ; A61K 39 / 3955 ANTIBODIES ( 2013 .01 ) ; A61K 31 /4706 ( 2013 .01 ) ; A61K 31 / 165 ( 2013 .01 ) ; CO7K 2317 /21 (2013 . 01 ) ; (71 ) Applicant: Eli D Ehrenpreis , Skokie , IL (US ) CO7K 2317/ 24 ( 2013. 01 ) ; A61K 2039/ 505 ( 2013 .01 ) (72 ) Inventor : Eli D Ehrenpreis, Skokie , IL (US ) (57 ) ABSTRACT Disclosed are methods for enhancing the efficacy of mono (21 ) Appl. No. : 15 /605 ,212 clonal antibody therapy , which entails co - administering a therapeutic monoclonal antibody , or a functional fragment (22 ) Filed : May 25 , 2017 thereof, and an effective amount of colchicine or hydroxy chloroquine , or a combination thereof, to a patient in need Related U . S . Application Data thereof . Also disclosed are methods of prolonging or increasing the time a monoclonal antibody remains in the (63 ) Continuation - in - part of application No . 14 / 947 , 193 , circulation of a patient, which entails co - administering a filed on Nov. 20 , 2015 . therapeutic monoclonal antibody , or a functional fragment ( 60 ) Provisional application No . 62/ 082, 682 , filed on Nov . of the monoclonal antibody , and an effective amount of 21 , 2014 . colchicine or hydroxychloroquine , or a combination thereof, to a patient in need thereof, wherein the time themonoclonal antibody remains in the circulation ( e . g . , blood serum ) of the Publication Classification patient is increased relative to the same regimen of admin (51 ) Int . -

Clinical Research Newsletter for Colleagues in the Community
Clinical Research Newsletter for Colleagues in the Community Welcome to the Winter 2017 issue of the Stanford Cancer surgery, robotic surgery, stereotactic radiosurgery such as Institute Clinical Research Newsletter. This quarterly CyberKnife®, microvascular reconstruction, intraoperative publication is designed to inform our colleagues in the radiation therapy (IORT), along with new chemotherapy trials. medical community, and especially physicians who are The Stanford Cancer Institute Neuro-Oncology Program considering treatment options for their patients with offers Phase I through III trials as well as multidisciplinary, cancer, about current clinical trials available at the Stanford collaborative evaluation and treatment of patients with tumors Cancer Institute, a National Cancer Institute designated of the nervous system. This includes, but is not restricted Comprehensive Cancer Center. Many of these trials provide to; brain metastases, leptomeningeal cancer, glioblastomas access to novel therapies including new “targeted” agents, and less aggressive gliomas, benign brain and spinal tumors, often not available in the community. and base of brain neoplasms including pituitary disorders. As leaders of the Stanford Head and Neck Cancer Care Clinical trials have focused on vaccine therapy, antibody Program, we are delighted to introduce this edition of the therapy, novel chemotherapy agents, radiation sensitizers, newsletter, as it focuses on our Neuro-Oncology, Thoracic novel radiation therapy, and radiosurgery techniques. Oncology, and Developmental Therapeutics programs. The Developmental Therapeutics Program conducts Each of these programs offers cutting-edge clinical trials pharmacokinetic and pharmacodynamic driven first-in- for patients with tumors that can be challenging to treat human trials tailored to make early, informed decisions with current routine care. Weekly multidisciplinary tumor regarding the suitability of novel molecular agents for boards are available for each program. -

(12) Patent Application Publication (10) Pub. No.: US 2017/0172932 A1 Peyman (43) Pub
US 20170172932A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2017/0172932 A1 Peyman (43) Pub. Date: Jun. 22, 2017 (54) EARLY CANCER DETECTION AND A 6LX 39/395 (2006.01) ENHANCED IMMUNOTHERAPY A61R 4I/00 (2006.01) (52) U.S. Cl. (71) Applicant: Gholam A. Peyman, Sun City, AZ CPC .......... A61K 9/50 (2013.01); A61K 39/39558 (US) (2013.01); A61K 4I/0052 (2013.01); A61 K 48/00 (2013.01); A61K 35/17 (2013.01); A61 K (72) Inventor: sham A. Peyman, Sun City, AZ 35/15 (2013.01); A61K 2035/124 (2013.01) (21) Appl. No.: 15/143,981 (57) ABSTRACT (22) Filed: May 2, 2016 A method of therapy for a tumor or other pathology by administering a combination of thermotherapy and immu Related U.S. Application Data notherapy optionally combined with gene delivery. The combination therapy beneficially treats the tumor and pre (63) Continuation-in-part of application No. 14/976,321, vents tumor recurrence, either locally or at a different site, by filed on Dec. 21, 2015. boosting the patient’s immune response both at the time or original therapy and/or for later therapy. With respect to Publication Classification gene delivery, the inventive method may be used in cancer (51) Int. Cl. therapy, but is not limited to such use; it will be appreciated A 6LX 9/50 (2006.01) that the inventive method may be used for gene delivery in A6 IK 35/5 (2006.01) general. The controlled and precise application of thermal A6 IK 4.8/00 (2006.01) energy enhances gene transfer to any cell, whether the cell A 6LX 35/7 (2006.01) is a neoplastic cell, a pre-neoplastic cell, or a normal cell. -

Selectively Targeting TGF-Β with Trabedersen/OT-101 in Treatment of Evolving and Mild ARDS in COVID-19
Review Article CLINICAL INVESTIGATION Selectively targeting TGF-β with Trabedersen/OT-101 in treatment of evolving and mild ARDS in COVID-19 Abstract Fatih M Uckun1,2*, Based on the role of TGF- β in the immunopathology of ARDS, we and others have proposed the use of Larn Hwang1, Vuong Trieu1 TGF-β inhibitors for the treatment of COVID-19 pneumonia and ARDS. TGF-targeting is employed as a strategy to stimulate the immune system of advanced-stage cancer patients in an attempt to overcome the 1Immuno-Oncology Program, Oncotelic immunosuppression and T-cell exhaustion within the tumor microenvironment. Nevertheless, we do not Inc., Agoura Hills, CA 91301, USA anticipate any worsening of existing ARDS or Cytokine Storm/Cytokine Release Syndrome (CRS) of COVID-19 patients as a treatment-emergent complication with our contemplated use of the anti-TGF-β RNA therapeutic 2COVID-19 Task Force, Worldwide Clinical OT-101. That is because (i) inhibitors of TGF-β signaling are not associated with ARDS, Cytokine Storm/CRS, Trials, Wayne, PA 19087, USA or systemic capillary leak, (ii) OT-101 did not cause any pulmonary toxicity, non-infectious pneumonitis, CRS, systemic or pulmonary capillary leak or ARDS in any of the 61 patients with advanced solid tumors enrolled *Author for correspondence: in Phase I/II study (ClinicalTrials.gov identifier: NCT00844064) who received much longer periods of OT-101 [email protected] therapy, and (iii) OT-101 did not cause in human subjects an elevation of TNF-α, IL-6 or IL-10 levels associated with CRS and ARDS in COVID-19 patients-likewise, OT-101 did not induce production of these inflammatory cytokines in cultures of human white blood cells. -

Recommended INN: List 63
WHO Drug Information, Vol. 24, No. 1, 2010 Recommended INN: List 63 International Nonproprietary Names for Pharmaceutical Substances (INN) RECOMMENDED International Nonproprietary Names: List 63 Notice is hereby given that, in accordance with paragraph 7 of the Procedure for the Selection of Recommended International Nonproprietary Names for Pharmaceutical Substances [Off. Rec. Wld Health Org., 1955, 60, 3 (Resolution EB15.R7); 1969, 173, 10 (Resolution EB43.R9); Resolution EB115.R4 (EB115/2005/ERC/1)], the following names are selected as Recommended International Nonproprietary Names. The inclusion of a name in the lists of Recommended International Nonproprietary Names does not imply any recommendation of the use of the substance in medicine or pharmacy. Lists of Proposed (1–101) and Recommended (1–62) International Nonproprietary Names can be found in Cumulative List No. 13, 2009 (available in CD-ROM only). Dénominations communes internationales des Substances pharmaceutiques (DCI) Dénominations communes internationales RECOMMANDÉES: Liste 63 Il est notifié que, conformément aux dispositions du paragraphe 7 de la Procédure à suivre en vue du choix de Dénominations communes internationales recommandées pour les Substances pharmaceutiques [Actes off. Org. mond. Santé, 1955, 60, 3 (résolution EB15.R7); 1969, 173, 10 (résolution EB43.R9); résolution EB115.R4 (EB115/2005/ERC/1)] les dénominations ci-dessous sont choisies par l’Organisation mondiale de la Santé en tant que dénominations communes internationales recommandées. L’inclusion d’une dénomination dans les listes de DCI recommandées n’implique aucune recommandation en vue de l’utilisation de la substance correspondante en médecine ou en pharmacie. On trouvera d’autres listes de Dénominations communes internationales proposées (1–101) et recommandées (1–62) dans la Liste récapitulative No. -
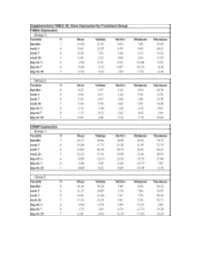
Fresolimumab Suppl Methods
Supplementary Figure 1. Fresolimumab treated patients show decreased biomarkers and skin scores. Graphs show thrombopondin-1 (THBS1, panels a and b) and cartilage oligomeric protein (COMP, panels c and d) gene expression, and MRSS (panels e and f) from study patients in group 1, receiving two doses of 1 mg/kg fresolimumab, (a, c, and e) and group 2, receiving one dose of 5 mg/kg fresolimumab (b, d and f). Line graphs show changes in individual patients over time. A B C Supplementary Figure 2: Biomarker gene expression correlates with the local skin score. The local forearm skin score correlates highly with the MRSS (panel a), as well as THBS1 (panel b) and COMP (panel c) gene expression. Supplementary Figure 3. Autoantibody levels measured at baseline and 11 weeks after fresolimumab treatment. Anti-RNA polymerase III levels for anti-RNA polymerase III- positive patients are shown in blue. Anti-Scl70 levels for anti-Scl70-positive patients are shown in red. Autoantibody levels in two of the four anti-Scl70-positive patients lie on top of the other two. Supplementary Figure 4. Disease duration compared to the change in MRSS 7 weeks after fresolimumab treatment. The change in MRSS after fresolimumab treatment showed no correlation with the disease duration (r2 = 0.00063) Supplementary Figure 5A. Immunohistochemical Supplementary Figure 5B. Skin collagen staining of plasminogen activator protein-1 (PAI- thickness. Based on trichrome staining, dermal 1/serpine1). Each patient is represented by a thickness was measured from the dermal-epidermal different color/style of marker. The median of the junction to subcutaneous fat.