( -Phenyl- -Aminobutyric Acid) Dependence And
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Baclofen Overdose D
Postgraduate Medical Journal (February 1980) 56, 108-109 Postgrad Med J: first published as 10.1136/pgmj.56.652.108 on 1 February 1980. Downloaded from Baclofen overdose D. J. LIPSCOMB* T. J. MEREDITHt M.B.. M.R.C.P. M.A., M.R.C.P. *Department of Medicine, Peterborough District Hospital, Peterborough PE3 6DA, and tPoisons Unit, Guy's Hospital, London SE] 9RT Summary Case report A 57-year-old woman suffering from multiple sclerosis A 51-year-old woman, who had suffered from took an estimated 1500 mg of baclofen. She became multiple sclerosis for 15 years, was prescribed baclo- deeply unconscious with generalized flaccid muscle fen 40 mg daily as part of her treatment for spastic paralysis and absent tendon reflexes. Toxicological paraplegia. One morning, she was found lying in analysis confirmed the presence of baclofen together bed unconscious and was admitted to hospital. with small amounts of paracetamol and glutethimide. Information given by her husband suggested that an Supportive therapy, including assisted ventilation for overdose of baclofen (consisting of 152 10-mg 3 days, led to complete recovery; anticonvulsant tablets) had been taken about 2 5 hr before being drugs were necessary for the treatment of grand mal found unconscious. On arrival in hospital 90 min fits. The clinical features and treatment of baclofen later, she was deeply unconscious and unresponsive overdose are discussed. to painful stimuli. Respiration was depressed and the cough reflex was absent. She was intubated without Introduction resistance and ventilated; gastric lavage was per-Protected by copyright. Baclofen (Lioresal, Ciba) is widely used in the formed but no tablets were returned in the lavage treatment of muscle spasticity. -

The Use of Intravenous Baclofen As Therapy for the Γ-Hydroxybutyric Acid Withdrawal Syndrome
Research Article Remedy Open Access Published: 12 Jun, 2017 The Use of Intravenous Baclofen as Therapy for the γ-hydroxybutyric Acid Withdrawal Syndrome Marc Sabbe1*, Francis Desmet1 and Sabrina Dewinter2 1Department of Emergency Medicine, University Hospitals, Catholic University of Leuven, Belgium 2Department of Pharmacy, University Hospitals, Catholic University of Leuven, Belgium Abstract Introduction: In this case series with three patients, we introduced baclofen, a γ-aminobutyric acid type B(GABA-B) receptor agonist, for treatment of the γ-hydroxybutyric acid (GHB) withdrawal syndrome. Materials and Methods: Single center case series performed on three patientswith a GHB withdrawal syndrome. They initially received massive doses of benzodiazepines, without sufficient effect. Two patients also received an unsuccessful continuous dexmedetomidine drip. In all patients, intravenous baclofen was started, with an intravenous loading dose between 0.5 and 2 milligrams (mg) to achieve a therapeutic level. Thereafter a continuous intravenous dose between 0.5 and 1 mg per hour for 12 h was administered to maintain a steady state. After that, baclofen was substituted orally with a daily oral dose varying between 20 mg and 40 mg which could be downgraded and stopped over the next days. They all continued to receive a standard benzodiazepine regimen during the baclofen trial. Results and Discussion: Main outcome measurements were the degree of withdrawal symptoms and the need for benzodiazepines during baclofen treatment. In all patients, a significant reduction of the GHB withdrawal syndrome was noted. A standard daily regimen of baseline benzodiazepine dosing between 40 mg and 80 mg diazepam was sufficient. Adverse effects of baclofen use were absent. -

Neurontin (Gabapentin)
Texas Prior Authorization Program Clinical Criteria Drug/Drug Class Gabapentin Clinical Criteria Information Included in this Document Neurontin (gabapentin) • Drugs requiring prior authorization: the list of drugs requiring prior authorization for this clinical criteria • Prior authorization criteria logic: a description of how the prior authorization request will be evaluated against the clinical criteria rules • Logic diagram: a visual depiction of the clinical criteria logic • Supporting tables: a collection of information associated with the steps within the criteria (diagnosis codes, procedure codes, and therapy codes); provided when applicable • References: clinical publications and sources relevant to this clinical criteria Note: Click the hyperlink to navigate directly to that section. Gralise (gabapentin Extended Release) • Drugs requiring prior authorization: the list of drugs requiring prior authorization for this clinical criteria • Prior authorization criteria logic: a description of how the prior authorization request will be evaluated against the clinical criteria rules • Logic diagram: a visual depiction of the clinical criteria logic • Supporting tables: a collection of information associated with the steps within the criteria (diagnosis codes, procedure codes, and therapy codes); provided when applicable • References: clinical publications and sources relevant to this clinical criteria Note: Click the hyperlink to navigate directly to that section. March 29, 2019 Copyright © 2019 Health Information Designs, LLC 1 Horizant -

GABA Receptors
D Reviews • BIOTREND Reviews • BIOTREND Reviews • BIOTREND Reviews • BIOTREND Reviews Review No.7 / 1-2011 GABA receptors Wolfgang Froestl , CNS & Chemistry Expert, AC Immune SA, PSE Building B - EPFL, CH-1015 Lausanne, Phone: +41 21 693 91 43, FAX: +41 21 693 91 20, E-mail: [email protected] GABA Activation of the GABA A receptor leads to an influx of chloride GABA ( -aminobutyric acid; Figure 1) is the most important and ions and to a hyperpolarization of the membrane. 16 subunits with γ most abundant inhibitory neurotransmitter in the mammalian molecular weights between 50 and 65 kD have been identified brain 1,2 , where it was first discovered in 1950 3-5 . It is a small achiral so far, 6 subunits, 3 subunits, 3 subunits, and the , , α β γ δ ε θ molecule with molecular weight of 103 g/mol and high water solu - and subunits 8,9 . π bility. At 25°C one gram of water can dissolve 1.3 grams of GABA. 2 Such a hydrophilic molecule (log P = -2.13, PSA = 63.3 Å ) cannot In the meantime all GABA A receptor binding sites have been eluci - cross the blood brain barrier. It is produced in the brain by decarb- dated in great detail. The GABA site is located at the interface oxylation of L-glutamic acid by the enzyme glutamic acid decarb- between and subunits. Benzodiazepines interact with subunit α β oxylase (GAD, EC 4.1.1.15). It is a neutral amino acid with pK = combinations ( ) ( ) , which is the most abundant combi - 1 α1 2 β2 2 γ2 4.23 and pK = 10.43. -

Phenibut Overdose in Combination with Fasoracetam: Emerging Drugs of Abuse
Open Access Journal of Clinical Intensive Care and Medicine Case Report Phenibut Overdose in Combination ISSN with Fasoracetam: Emerging Drugs 2639-6653 of Abuse Cristian Merchan1*, Ryan Morgan1, John Papadopoulos1,2 and David Fridman2 1Department of Pharmacy, New York University Langone Medical Center, New York, USA 2New York University School of Medicine, New York, USA *Address for Correspondence: Dr. Cristian INTRODUCTION D Merchan, Pharm.D, BCCCP, Critical Care Pharmacotherapist, Department of Pharmacy, The widespread availability of non-traditional dietary supplements and New York University Langone Medical Center, pharmacologically active substances via the Internet continues to introduce New York, USA, Tel: 516-263-1671; Email: [email protected] mechanisms for inadvertent toxidromes not commonly seen. Consumers are virtually unrestricted in their ability to acquire products purporting augmentation of normal Submitted: 25 October 2016 Approved: 15 December 2016 physiology for the purposes of enhancement, recreation, and/or potential abuse. The Published: 17 December 2016 safety proiles at standard or toxic doses remain largely unknown for many agents that Copyright: 2016 Merchan et al. This is an can be purchased electronically. We report a case of mixed toxicity related to phenibut open access article distributed under the and fosaracetam, both of which are readily available for consumer purchase from Creative Commons Attribution License, which online retailers. Written and verbal consent was obtained for this case presentation. permits unrestricted use, distribution, and reproduction in any medium, provided the CASE REPORT original work is properly cited. Keywords: Nootropic; GABA agonist; Drug of A 27-year-old male with a past medical history of anxiety was discovered abuse; Dietary supplement; Alternative medicine unresponsive on the sidewalk. -

Big Pain Assays Aren't a Big Pain with the Raptor Biphenyl LC Column
Featured Application: 231 Pain Management and Drugs of Abuse Compounds in under 10 Minutes by LC-MS/MS Big Pain Assays Aren’t a Big Pain with the Raptor Biphenyl LC Column • 231 compounds, 40+ isobars, 10 drug classes, 22 ESI- compounds in 10 minutes with 1 column. • A Raptor SPP LC column with time-tested Restek Biphenyl selectivity is the most versatile, multiclass-capable LC column available. • Achieve excellent separation of critical isobars with no tailing peaks. • Run fast and reliable high-throughput LC-MS/MS analyses with increased sensitivity using simple mobile phases. The use of pain management drugs is steadily increasing. As a result, hospital and reference labs are seeing an increase in patient samples that must be screened for a wide variety of pain management drugs to prevent drug abuse and to ensure patient safety and adherence to their medication regimen. Thera- peutic drug monitoring can be challenging due to the low cutoff levels, potential matrix interferences, and isobaric drug compounds. To address these chal- lenges, many drug testing facilities are turning to liquid chromatography coupled with mass spectrometry (LC-MS/MS) for its increased speed, sensitivity, and specificity. As shown in the analysis below, Restek’s Raptor Biphenyl column is ideal for developing successful LC-MS/MS pain medication screening methodologies. With its exceptionally high retention and unique selectivity, 231 multiclass drug compounds and metabolites—including over 40 isobars—can be analyzed in just 10 minutes. In addition, separate panels have been optimized on the Raptor Biphenyl column specifically for opioids, antianxiety drugs, barbiturates, NSAIDs and analgesics, antidepressants, antiepileptics, antipsychotics, hallucinogens, and stimulants for use during confirmation and quantitative analyses. -

Chapter 25 Mechanisms of Action of Antiepileptic Drugs
Chapter 25 Mechanisms of action of antiepileptic drugs GRAEME J. SILLS Department of Molecular and Clinical Pharmacology, University of Liverpool _________________________________________________________________________ Introduction The serendipitous discovery of the anticonvulsant properties of phenobarbital in 1912 marked the foundation of the modern pharmacotherapy of epilepsy. The subsequent 70 years saw the introduction of phenytoin, ethosuximide, carbamazepine, sodium valproate and a range of benzodiazepines. Collectively, these compounds have come to be regarded as the ‘established’ antiepileptic drugs (AEDs). A concerted period of development of drugs for epilepsy throughout the 1980s and 1990s has resulted (to date) in 16 new agents being licensed as add-on treatment for difficult-to-control adult and/or paediatric epilepsy, with some becoming available as monotherapy for newly diagnosed patients. Together, these have become known as the ‘modern’ AEDs. Throughout this period of unprecedented drug development, there have also been considerable advances in our understanding of how antiepileptic agents exert their effects at the cellular level. AEDs are neither preventive nor curative and are employed solely as a means of controlling symptoms (i.e. suppression of seizures). Recurrent seizure activity is the manifestation of an intermittent and excessive hyperexcitability of the nervous system and, while the pharmacological minutiae of currently marketed AEDs remain to be completely unravelled, these agents essentially redress the balance between neuronal excitation and inhibition. Three major classes of mechanism are recognised: modulation of voltage-gated ion channels; enhancement of gamma-aminobutyric acid (GABA)-mediated inhibitory neurotransmission; and attenuation of glutamate-mediated excitatory neurotransmission. The principal pharmacological targets of currently available AEDs are highlighted in Table 1 and discussed further below. -
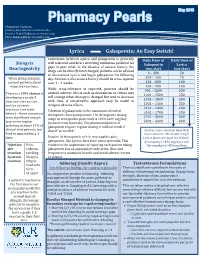
Lyrica Gabapentin: an Easy Switch!
Pharmacist Contacts: [email protected]; [email protected]; [email protected] Lyrica Gabapentin: An Easy Switch! Conversion between Lyrica and gabapentin is generally Daily Dose of Daily Dose of Shingrix well tolerated and direct switching minimizes potential for Gabapentin Lyrica Reactogenicity gaps in pain relief. In the absence of seizure history, the (mg/day) (mg/day) drugs can be directly interchanged; patients can be advised 0 – 300 50 to discontinue Lyrica and begin gabapentin the following When giving Shingrix, day. Patients with a seizure history should be cross-tapered 301 – 450 75 counsel patients about over 1 – 4 weeks. 451 – 600 100 expected reactions. 601 – 900 150 While cross-tolerance is expected, patients should be 901 – 1200 200 advised adverse effects such as drowsiness or edema may There is a 10% chance of 1201 – 1500 250 still emerge when therapy is changed but tend to decrease developing a grade 3 1501 – 1800 300 injection site reaction with time. A conservative approach may be useful to 1801 – 2100 350 and/or systemic mitigate adverse effects. reactions (see table 2101 – 2400 400 Titration of gabapentin to the maximum tolerated 2401 – 2700 450 below) – these symptoms therapeutic dose is important. The therapeutic dosing 2701 – 3000 500 were significant enough range in neuropathic pain trials is 1800-3600 mg/day to prevent regular (normal renal function). The pharmacokinetics of 3001 – 3600 600 activities in about 17% of gabapentin require regular dosing, it will not work if clinical trial patients, but dosed “as needed.” Studies show minimal benefit & tend to pass within 2-3 more adverse effects when high days. -

Conjugation of Tryptamine to Biologically Active Carboxylic Acids in Order to Create Prodrugs
ISSN 2380-5064 | The Arsenal is published by the Augusta University Libraries | http://guides.augusta.edu/arsenal Volume 4, Issue 1 (2021) Special Edition Issue CONJUGATION OF TRYPTAMINE TO BIOLOGICALLY ACTIVE CARBOXYLIC ACIDS IN ORDER TO CREATE PRODRUGS Colin Miller, Jr. and Iryna O. Lebedyeva Citation Miller, C., Jr., & Lebedyeva, I. O. (2021). Conjugation of tryptamine to biologically active carboxylic acids in order to create prodrugs. The Arsenal: The Undergraduate Research Journal of Augusta University, 4(1), 26. http://doi.org/10.21633/issn.2380.5064/s.2021.04.01.26 © Miller, Jr. and Lebedyeva 2021. This open access article is distributed under a Creative Commons Attribution NonCommercial-NoDerivs 2.0 Generic License (https://creativecommons.org/licenses/by-nc-nd/2.0/). Conjugation of Tryptamine to Biologically Active Carboxylic Acids in Order to Create Prodrugs Presenter(s): Colin Miller, Jr. Author(s): Colin Miller Jr. and Iryna O. Lebedyeva Faculty Sponsor(s): Iryna O. Lebedyeva, PhD Affiliation(s): Department of Chemistry and Physics (Augusta Univ.) ABSTRACT A number of blood and brain barrier penetrating neurotransmitters contain polar functional groups. Gamma-aminobutyric acid (GABA) is an amino acid, which is one of the primary inhibitory neurotransmitter in the brain and a major inhibitory neurotransmitter in the spinal cord. Antiepileptic medications such as Gabapentin, Phenibut, and Pregabalin have been developed to structurally represent GABA. These drugs are usually prescribed for the treatment of neuropathic pain. Since these drugs contain a polar carboxylic acid group, it affects their ability to penetrate the blood and brain barrier. To address the low bioavailability and tendency for intramolecular cyclization of Gabapentin, its less polar prodrug Gabapentin Enacarbil has been approved in 2011. -

Comparing Adverse Effects of Analgesic Strategies For
Comparing Adverse Effects daily and weekly time during which higher than that of the only ATC opi- their pain interfered with their mood oids group and 17 times higher than of Analgesic Strategies for or activities, and they indicated the that of the only PRN opioids group. Chronic Cancer Pain amount of relief they received from Source: J Pain Symptom Manage. 2007;33(1):67–77. their pain medicine in the previ- Nausea, vomiting, drowsiness, lack ous week. They also completed the of energy, urinary retention—some- Karnofsky Performance Status (KPS), Clopidogrel: Best Results times the adverse effects of the anal- which measures patients’ ability to per- Pre- or Post-PCI? gesic medication are enough to derail form activities of daily living and their pain management for patients with need for caregiver assistance. When is the best time to give the anti- cancer. It would help to understand There were no significant differ- platelet clopidogrel to patients sched- the relationships between the type of ences between any of the four groups uled for diagnostic coronary angiogra- analgesic prescription and the preva- in terms of pain intensity or amount of phy—before ad hoc coronary stenting lence and severity of adverse effects. time spent in pain. Total pain interfer- or immediately after? Researchers from But not much information is available, ence scores, however, were significantly University of Debrecen, Debrecen, say researchers from the University higher in the ATC plus PRN opioids Hungary and Medical University of of California, San Francisco; the Uni- group than in the no opioids group. Vienna and Wilhelminenhospital, both versity of Nebraska, Omaha; and the The ATC plus PRN opioids group in Vienna, Austria say starting treat- University of Texas Southwestern Med- also had significantly lower functional ment before percutaneous coronary ical Center, Dallas. -

Baclofen and Other GABAB Receptor Agents Are Allosteric Modulators of the CXCL12 Chemokine Receptor CXCR4
The Journal of Neuroscience, July 10, 2013 • 33(28):11643–11654 • 11643 Cellular/Molecular Baclofen and Other GABAB Receptor Agents Are Allosteric Modulators of the CXCL12 Chemokine Receptor CXCR4 Alice Guyon,1,2,3 Amanda Kussrow,4 Ian Roys Olmsted,4 Guillaume Sandoz,1,2,5 Darryl J. Bornhop,4* and Jean-Louis Nahon1,2,6* 1Universite´ de Nice Sophia Antipolis, 06103 Nice, France, 2Centre National de la Recherche Scientifique (CNRS), Institut de Pharmacologie Mole´culaire et Cellulaire, UMR 7275, 06560 Valbonne, France, 3Department of Molecular and Cellular Biology and Helen Wills Neuroscience Institute, University of California, Berkeley, Berkeley, California 94720, 4Vanderbilt Institute of Chemical Biology, Nashville, Tennessee 37235-1822, 5Institute of Biology Valrose, CNRS UMR 7277, INSERM UMR 1091, Laboratories of Excellence, Ion Channel Science and Therapeutics, 06103 Nice, France, 6Station de Primatologie—UPS 846—Centre CNRS, RD56, 13790 Rousset sur Arc, France CXCR4, a receptor for the chemokine CXCL12 (stromal-cell derived factor-1␣), is a G-protein-coupled receptor (GPCR), expressed in the immune and CNS and integrally involved in various neurological disorders. The GABAB receptor is also a GPCR that mediates metabotropic action of the inhibitory neurotransmitter GABA and is located on neurons and immune cells as well. Using diverse approaches, we report novel interaction between GABAB receptor agents and CXCR4 and demonstrate allosteric binding of these agents to CXCR4. First, both GABAB antagonistsandagonistsblockCXCL12-elicitedchemotaxisinhumanbreastcancercells.Second,aGABAB antagonistblocksthepotentiationby CXCL12ofhigh-thresholdCa 2ϩ channelsinratneurons.Third,electrophysiologyinXenopusoocytesandhumanembryonickidneycellline293 ϩ cells in which we coexpressed rat CXCR4 and the G-protein inward rectifier K (GIRK) channel showed that GABAB antagonist and agonist modified CXCL12-evoked activation of GIRK channels. -

Membrane Stabilizer Medications in the Treatment of Chronic Neuropathic Pain: a Comprehensive Review
Current Pain and Headache Reports (2019) 23: 37 https://doi.org/10.1007/s11916-019-0774-0 OTHER PAIN (A KAYE AND N VADIVELU, SECTION EDITORS) Membrane Stabilizer Medications in the Treatment of Chronic Neuropathic Pain: a Comprehensive Review Omar Viswanath1,2,3 & Ivan Urits4 & Mark R. Jones4 & Jacqueline M. Peck5 & Justin Kochanski6 & Morgan Hasegawa6 & Best Anyama7 & Alan D. Kaye7 Published online: 1 May 2019 # Springer Science+Business Media, LLC, part of Springer Nature 2019 Abstract Purpose of Review Neuropathic pain is often debilitating, severely limiting the daily lives of patients who are affected. Typically, neuropathic pain is difficult to manage and, as a result, leads to progression into a chronic condition that is, in many instances, refractory to medical management. Recent Findings Gabapentinoids, belonging to the calcium channel blocking class of drugs, have shown good efficacy in the management of chronic pain and are thus commonly utilized as first-line therapy. Various sodium channel blocking drugs, belonging to the categories of anticonvulsants and local anesthetics, have demonstrated varying degrees of efficacy in the in the treatment of neurogenic pain. Summary Though there is limited medical literature as to efficacy of any one drug, individualized multimodal therapy can provide significant analgesia to patients with chronic neuropathic pain. Keywords Neuropathic pain . Chronic pain . Ion Channel blockers . Anticonvulsants . Membrane stabilizers Introduction Neuropathic pain, which is a result of nervous system injury or lives of patients who are affected. Frequently, it is difficult to dysfunction, is often debilitating, severely limiting the daily manage and as a result leads to the progression of a chronic condition that is, in many instances, refractory to medical This article is part of the Topical Collection on Other Pain management.