Emergency Care Issues in Hemophilia
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

WHO Guidance on Management of Snakebites
GUIDELINES FOR THE MANAGEMENT OF SNAKEBITES 2nd Edition GUIDELINES FOR THE MANAGEMENT OF SNAKEBITES 2nd Edition 1. 2. 3. 4. ISBN 978-92-9022- © World Health Organization 2016 2nd Edition All rights reserved. Requests for publications, or for permission to reproduce or translate WHO publications, whether for sale or for noncommercial distribution, can be obtained from Publishing and Sales, World Health Organization, Regional Office for South-East Asia, Indraprastha Estate, Mahatma Gandhi Marg, New Delhi-110 002, India (fax: +91-11-23370197; e-mail: publications@ searo.who.int). The designations employed and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of the World Health Organization concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. Dotted lines on maps represent approximate border lines for which there may not yet be full agreement. The mention of specific companies or of certain manufacturers’ products does not imply that they are endorsed or recommended by the World Health Organization in preference to others of a similar nature that are not mentioned. Errors and omissions excepted, the names of proprietary products are distinguished by initial capital letters. All reasonable precautions have been taken by the World Health Organization to verify the information contained in this publication. However, the published material is being distributed without warranty of any kind, either expressed or implied. The responsibility for the interpretation and use of the material lies with the reader. In no event shall the World Health Organization be liable for damages arising from its use. -

Bruise, Contusion & Ecchymosis Conventions
Bruise, Contusion and Ecchymosis MedDRA Proactivity Proposal Implementation MedDRA Version 16.0 I. MSSO Recognized Definitions of Concepts and Terms The MSSO has designated Dorland’s Illustrated Medical Dictionary as the standard reference for medical definitions. The following definitions are cited from Dorland’s 27th edition: Bruise – A superficial injury produced by impact without laceration; a contusion Contusion – A bruise; an injury of a part without a break in the skin Ecchymosis – A small hemorrhagic spot, larger than a petechia, in the skin or mucous membrane forming a nonelevated, rounded or irregular, blue or purplish patch. Hematoma – A localized collection of blood, usually clotted, in an organ, space, or tissue, due to a break in the wall of a blood vessel. Hemorrhage – The escape of blood from the vessels; bleeding. Petechia – A pinpoint, non-raised, perfectly round, purplish red spot caused by intradermal or submucous hemorrhage. Additional comments regarding the definitions: Bruise and contusion are synonymous, and are often used in a colloquial context. Bruise and contusion are each considered a result of injury. Bruise and contusion have been used to describe minor hemorrhage within tissue, where traumatized blood vessels leak blood into the interstitial space. Commonly, capillaries and sometimes venules are injured within skin, subcutaneous tissue, muscle, or bone. In addition to trauma, the terms bruise, ecchymosis, and to a lesser extent, contusion, have also been used as clinical signs of disorders of platelet function, coagulopathies, venous congestion, allergic reactions, etc. Hemorrhage may be used to describe blood escaping from vessels and retained in the interstitial space, and perhaps more commonly, to describe the escape of blood from vessels, and flowing freely external to the tissues. -

Grey Turner's and Cullen's Signs Induced by Spontaneous Hemorrhage of the Abdominal Wall After Coughing
CASE REPORT pISSN 2288-6575 • eISSN 2288-6796 https://doi.org/10.4174/astr.2017.93.2.115 Annals of Surgical Treatment and Research Grey Turner’s and Cullen’s signs induced by spontaneous hemorrhage of the abdominal wall after coughing Zhe Fan, Yingyi Zhang Department of General Surgery, The Third People’s Hospital of Dalian, Dalian Medical University, Dalian, China Grey Turner’s and Cullen’s signs are rare clinical signs, which most appear in patients with severe acute pancreatitis. The present patient complained of abdominal pain after coughing. However, contrast-enhanced CT revealed a hemorrhage of the abdominal wall. Therefore, spontaneous hemorrhage of the abdominal wall was diagnosed. The patient recovered through immobilization and hemostasis therapy. This case report and literature review aims to remind clinicians of manifestations and treatment of spontaneous hemorrhage. [Ann Surg Treat Res 2017;93(2):115-117] Key Words: Grey Turner’s sign, Cullen’s sign, Spontaneous hemorrhage INTRODUCTION right thigh were noted (Fig. 2). All of the laboratory tests were normal, except that hemoglobin was decreased to 10.5 Grey Turner’s and Cullen’s signs generally suggest a large g/L. Contrastenhanced CT of the abdomen revealed a huge retroperitoneal hematoma, such as hemorrhagic pancreatitis. hematoma in the right lateral abdominal wall (Fig. 3). Therefore, The current case was unusual because of a spontaneous hemo Grey Turner’s and Cullen’s signs induced by spontaneous rrhage of the abdominal wall after coughing leading to Grey hemorrhage of the abdominal wall after cough were diagnosed. Turner’s and Cullen’s signs. -

Immune Thrombocytopenic Purpura in a Twin Girl Revealed by a Traumatic Injury in Parakou (North Benin)
Immune thrombocytopenic purpura in a twin girl revealed by a traumatic injury in parakou (North Benin) Adedemy JD 1*, Noudamadjo A 1, Kpanidja G 1, Agossou J 1, Agbeille Mohamed F 1, Dovonou CA 2 1 Mother and Child Department, Parakou Teaching Hospital, Republic of Benin, and Faculty of Medicine, University of Parakou West Africa 2 Department of Medicine, Parakou Teaching Hospital, and Faculty of Medicine, University of Parakou, West Africa Abstract Background: ITP seems to be rare but in tropical settings thrombocytopenia is often encountered among children. Objective: Authors through this case report are putting emphasy on the diagnosis and management of ITP in a 4 year old twin girl admitted in the pediatric emergency ward for hematuria and bleeding from various origins seen in the context of a domestic trauma. Results: The various clinical signs have been analyzed to confirm ITP through exclusion of other possible health conditions. The management of ITP depend on the severity of clinical signs and in some cases the situations can be life threatening. In this case report, Blood transfusion and corticosteroids were the main treatment tools. The hospital stay was about 47 days and an ambulatory follow up was conducted for almost 6 months. Conclusion: In the context of various bleeding disorders, hematuria and thrombocytopenia, autoimmune thrombocytopenia in a twin girl was revealed by a domestic trauma. Citation: Adedemy JD, Noudamadjo A, Kpanidja G, Agossou J, Agbeille MF, Dovonou CA (2019) Immune Thrombocytopenic Purpura in a twin girl revealed by a traumatic injury in Parakou (North Benin). Adv Pediatr Res 6:27. -

How Significant Is Bleeding in Antiphospholipid Antibody
Treatment of Anti-Phospholipid Syndrome and Prothrombin Deficiency with Plasma Exchange Lowell Tilzer KU Medical Center Department of Pathology & Lab Medicine Case 72 YEARS OLD FEMALE PRESENTED TO KUMC WITH BLEEDING FOLLOWING ROUTINE HEMORRHOIDECTOMY SURGERY Past Medical History • Surgeries: Bilateral tubal ligation, appendectomy, partial hysterectomy, bilateral bunion surgery – No bleeding complications • 2008: Presented to ER with chest pain – Incidentally found prolonged PT and PTT + Lupus anticoagulant and anticardiolipin antibodies • 2009: Melanotic stool with severe anemia (Hb 4) – Blood transfusion (>10 units) – Attributed to long-term use of Aspirin Brief course Initial Work-up Reported Normal Test Value Range PT/INR 2.2 (HIGH) 0.8-1.2 PTT 87.4 (HIGH) 24.0-40.0 PT mixing study @ 60 mins 1.5 (HIGH) PTT mixing study @ 60 mins 82.8 (HIGH) Factor 2 assay 10% (LOW) 50-150% Factor 5 assay 78% 50-150% Factor 7 assay 153% (HIGH) 50-150% Factor 8 assay 235% (HIGH) 50-150% Factor 10 assay 68% 50-150% 300 Linear dilutions 250 200 150 100 % Normal control 50 0 Factor 2 Factor 5 Factor 7 Factor 8 Factor 10 Additional coagulation study Test Result Interpretation dRVVT Prolonged Lupus anticoagulant Abs Hexagonal Lupus Positive Lupus anticoagulant Abs anticoagulant Anti-b2 GPI IgG Positive Anti-b2 GPI IgM Positive Support APS diagnosis Anti-cardiolipin IgG Positive Anti-cardiolipin IgM Positive There is NO Factor 2 inhibitor, therefore previous result of low Factor 2 inhibitor <0.4 BU Factor 2 level was due to lupus (activity-based) anticoagulant Abs against phospholipids in the assay. Algorithm for Screening tests (PT/INR, aPTT) coagulopathies Mixing study Heparin, DTI PL dependent assays (aPTT or dRVVT + Hexa) PTT PT PT PTT LA+ Factor LA- Intrinsic Common Extrinsic inhibitors pathway pathway pathway Revised Criteria for Antiphospholipid Syndrome (APS) (Sydney Criteria) APS: ≥ 1 Laboratory Criteria AND ≥ 1 Clinical Criteria • Laboratory Criteria: “≥ 2 occasions 3 months apart” 1. -

Retroperitoneal Hemorrhage – a Review of the Eponymous Cutaneous Signs
Journal of Pakistan Association of Dermatologists. 2020; 30(3): 501-506. Review Article Retroperitoneal hemorrhage – A review of the eponymous cutaneous signs Sajad Ahmad Salati Department of Surgery, Unaizah College of Medicine, Qassim University, Saudi Arabia. Abstract Physical diagnosis by eliciting clinical signs still plays an important role in the management of diseases .In case of retroperitoneal bleeding due to any cause, there are five important eponymous cutaneous signs mentioned in literature. These include Cullen’s sign, Turner’s sign, Fox’s sign, Bryant’s sign and Stabler’s sign. These signs are briefly reviewed in this article. Key words Retroperitoneal hemorrhage, Cullen’s sign, Turner’s sign, Fox’s sign, Bryant’s sign, Stabler’s sign. Introduction Web of Science, Semantic Scholar and Reseachgate after search on keywords: In recent years, there have been revolutionary retroperitoneal hemorrhage, skin sign, advances in imaging and diagnostic facilities. eponymous sign and diagnostic sign. The search But in spite of these changes, there are certain was extended across to the cross references. time tested objective physical findings that Only the articles published in English were retain their relevance and importance in eliciting included and no time limits were set. diagnosis or in narrowing the differential diagnosis. The eponymous cutaneous signs of Clinical presentation retroperitoneal hemorrhage are such clinical signs which aid in diagnosis and management of 1. Cullen's sign (Figure 1A) denotes patients besides highlighting the rich history of ecchymosis around the umbilical area as seen dermatology. These eponyms are based on the after retroperitoneal hemorrhage . This sign is physician who first described the clinical accredited to Thomas Stephen Cullen (1868- findings. -

Intracranial Haemorrhage in a 26 Year-Old Woman with Idiopathic Thrombocytopenic Purpura
Postgraduate Medical Journal (1987) 63, 781-783 Postgrad Med J: first published as 10.1136/pgmj.63.743.781 on 1 September 1987. Downloaded from Intracranial haemorrhage in a 26 year-old woman with idiopathic thrombocytopenic purpura Gavin Awerbuch and Reuven Sandyk Department ofNeurology, University ofArizona Health Sciences Center, Tucson, AZ. 85724, USA. Summary: Intracranial haemorrhage (ICH), a rare complication of idiopathic thrombocytopenic purpura (ITP), described only once previously in an adult, is usually fatal. We report a previously healthy 26 year old woman with chronic ITP in whom spontaneous ICH developed. The eventual favourable outcome in this case despite severe initial neurological deficit makes this case unusual. The importance of aggressive management in an ITP associated ICH is stressed and a plan for management is suggested. Introduction Although relatively rare, intracranial haemorrhage of 5.7 g/dl, white blood count of 15.5 x I09/l with a (ICH) is the most serious complication of idiopathic normal differential and a platelet count of 6.0 x 109/l, thrombocytopenic purpura (ITP) and is the leading reticulocyte count was 3.2%. The prothrombin time, reported cause ofdeath.'`3 In children, nearly 20 cases partial thromboplastin time, thrombin time, fibrin- Protected by copyright. of acute ITP complicated by ICH have been reported. ogen, fibrin monomers, and fibrin split products were Previous reports have alluded to ICH associated with all normal. An electroencephalogram (EEG), gallium ITP in adults,4`7 but ICH has been documented only scan of the abdomen, chest X-ray, and computerized once in adults.8 We report the occurrence of an axial tomography (CT scan) of the head were normal. -

Cerebral Venous Thrombosis After Intravenous Immunoglobulin Therapy in Immune Thrombocytopenic Purpura
Case Report Cerebral Venous Thrombosis after Intravenous Immunoglobulin Therapy in Immune Thrombocytopenic Purpura Joe James, P. V. Shiji, Chandni Radhakrishnan Department of Internal Medicine, Government Medical College, Kozhikode, Kerala, India Abstract A common misconception is that immune thrombocytopenic purpura (ITP) causes only bleeding diathesis. From this case vignette of a young male with ITP who had cerebral venous thrombosis, we highlight the importance of considering venous thrombosis in such patients when they present with focal cerebral signs. Keywords: Immune thrombocytopenic purpura, intravenous immunoglobulin, venous thrombosis 3 INTRODUCTION blood cell count of 21,800/mm with 84% neutrophils, 12% lymphocytes, and 4% mixed cells; hemoglobin of 11.2 g/dl; and Immune thrombocytopenic purpura (ITP) is a disorder platelet count of 65,000/mm3. Serum sodium was 136 mEq/L, characterized by autoimmune destruction of platelets, potassium 3.9 mEq/L, calcium 9.1 mg/dL, and plasma glucose which commonly presents as bleeding diathesis. However, 105 mg/dL. Serum creatinine was 1.1 mg/dL, total bilirubin thromboembolic complications can also occur in ITP. Here, was 1.5 mg/dL, and alanine aminotransferase was 30 IU. we describe a case of ITP who presented with cerebral venous International normalized ratio was 1.03 and activated partial thrombosis (CVT) after treatment with high‑dose intravenous thromboplastin time was 31.3 (reference <28). Anti‑nucleosome immunoglobulin (IVIg). antibody, anti ds‑DNA, C3 and C4 levels were normal. HIV, HBsAg, and HCV serology were negative. Computed CASE REPORT tomography (CT) of the head showed a small intraparenchymal A 26‑year‑old‑male with a history of ITP, presented with hematoma in the right frontal lobe [Figure 1a]. -

Protein C 결핍에서 발생한 소모성 응고질환으로 인한 뇌내출혈로 야기된 뇌성마비 환자에 대한 증례기록 연세대학교 의과대학 세브란스병원 재활의학과1, 소아청소년과2 조유나1・이영 목 2・박 은 숙 1・최 자 영 1・박 천 웅 1・나 동 욱 1
J Korean Child Neurol Soc 2017;25(1):44-47 Case report pISSN 1226-6884•eISSN 2383-8973 Protein C 결핍에서 발생한 소모성 응고질환으로 인한 뇌내출혈로 야기된 뇌성마비 환자에 대한 증례기록 연세대학교 의과대학 세브란스병원 재활의학과1, 소아청소년과2 조유나1・이영 목 2・박 은 숙 1・최 자 영 1・박 천 웅 1・나 동 욱 1 Cerebral Palsy due to Intracranial Hemorrhage Yoona Cho, MD1, Young-Mock Lee, MD2, Eun Sook Park, MD1, Ja Young Choi, MD1, Chunung Caused by Consumptive Coagulopathy in Protein C Park, MD1, Dong-wook Rha, MD, PhD1 Deficiency: A Case Report 1Department and Research Institute of Rehabi- Protein C (PROC) is a potent anticoagulant inactivating coagulation factors Va and litation Medicine, 2Department of Pediatrics, VIIIa. PROC deficiency is very rare condition inherited as an autosomal dominant Gangnam Severance Hospital, Yonsei University or recessive trait, and associated with various thromboembolic and ischemic College of Medicine, Seoul, Korea conditions. Moreover, severe form of PROC deficiency can cause fatal hemorrhagic complications due to consumptive coagulopathy. We reported two children with Submitted: 24 August, 2016 Revised: 13 September, 2016 hemorrhagic stroke who were diagnosed as severe PROC deficiency caused by two Accepted: 13 September, 2016 different types of compound heterozygous PROC gene mutations. We described results of laboratory tests, genetic analysis, brain magnetic resonance images, and Correspondence to Dong-wook Rha, MD, PhD functional outcomes. Both children received prophylactic anticoagulation therapy Department and Research Institute of Rehabilitation, and presented with purple-colored skin lesions during rehabilitation. Purpura Medicine Yonsei University College of Medicine, Severance Rehabilitation Hospital, BioMechanics and fulminans caused by insufficient anticoagulation should be differentiated from Robotic Rehabilitation Laboratory, 50-1 Yonsei-ro hematoma caused by excessive anticoagulation therapy in these children. -

Thrombocytopenia and Intracranial Venous Sinus Thrombosis After “COVID-19 Vaccine Astrazeneca” Exposure
Journal of Clinical Medicine Article Thrombocytopenia and Intracranial Venous Sinus Thrombosis after “COVID-19 Vaccine AstraZeneca” Exposure Marc E. Wolf 1,2 , Beate Luz 3, Ludwig Niehaus 4 , Pervinder Bhogal 5, Hansjörg Bäzner 1,2 and Hans Henkes 6,7,* 1 Neurologische Klinik, Klinikum Stuttgart, D-70174 Stuttgart, Germany; [email protected] (M.E.W.); [email protected] (H.B.) 2 Department of Neurology, Universitätsmedizin Mannheim, University of Heidelberg, D-68167 Mannheim, Germany 3 Zentralinstitut für Transfusionsmedizin und Blutspendedienst, Klinikum Stuttgart, D-70174 Stuttgart, Germany; [email protected] 4 Neurologie, Rems-Murr-Klinikum Winnenden, D-71364 Winnenden, Germany; [email protected] 5 Department of Interventional Neuroradiology, The Royal London Hospital, Barts NHS Trust, London E1 1FR, UK; [email protected] 6 Neuroradiologische Klinik, Klinikum Stuttgart, D-70174 Stuttgart, Germany 7 Medical Faculty, University Duisburg-Essen, D-47057 Duisburg, Germany * Correspondence: [email protected]; Fax: +49-711-278-345-09 Abstract: Background: As of 8 April 2021, a total of 2.9 million people have died with or from the coronavirus infection causing COVID-19 (Corona Virus Disease 2019). On 29 January 2021, the European Medicines Agency (EMA) approved a COVID-19 vaccine developed by Oxford University Citation: Wolf, M.E.; Luz, B.; and AstraZeneca (AZD1222, ChAdOx1 nCoV-19, COVID-19 vaccine AstraZeneca, Vaxzevria, Cov- Niehaus, L.; Bhogal, P.; Bäzner, H.; ishield). While the vaccine prevents severe course of and death from COVID-19, the observation of Henkes, H. Thrombocytopenia and pulmonary, abdominal, and intracranial venous thromboembolic events has raised concerns. Objec- Intracranial Venous Sinus Thrombosis after “COVID-19 Vaccine tive: To describe the clinical manifestations and the concerning management of patients with cranial AstraZeneca” Exposure. -

Modern Management of Traumatic Hemothorax
rauma & f T T o re l a t a m n r e u n o t J Mahoozi, et al., J Trauma Treat 2016, 5:3 Journal of Trauma & Treatment DOI: 10.4172/2167-1222.1000326 ISSN: 2167-1222 Review Article Open Access Modern Management of Traumatic Hemothorax Hamid Reza Mahoozi, Jan Volmerig and Erich Hecker* Thoraxzentrum Ruhrgebiet, Department of Thoracic Surgery, Evangelisches Krankenhaus, Herne, Germany *Corresponding author: Erich Hecker, Thoraxzentrum Ruhrgebiet, Department of Thoracic Surgery, Evangelisches Krankenhaus, Herne, Germany, Tel: 0232349892212; Fax: 0232349892229; E-mail: [email protected] Rec date: Jun 28, 2016; Acc date: Aug 17, 2016; Pub date: Aug 19, 2016 Copyright: © 2016 Mahoozi HR. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Abstract Hemothorax is defined as a bleeding into pleural cavity. Hemothorax is a frequent manifestation of blunt chest trauma. Some authors suggested a hematocrit value more than 50% for differentiation of a hemothorax from a sanguineous pleural effusion. Hemothorax is also often associated with penetrating chest injury or chest wall blunt chest wall trauma with skeletal injury. Much less common, it may be related to pleural diseases, induced iatrogenic or develop spontaneously. In the vast majority of blunt and penetrating trauma cases, hemothoraces can be managed by relatively simple means in the course of care. Keywords: Traumatic hemothorax; Internal chest wall; Cardiac Hemodynamic response injury; Clinical manifestation; Blunt chest-wall injuries; Blunt As above mentioned the hemodynamic response is a multifactorial intrathoracic injuries; Penetrating thoracic trauma response and depends on severity of hemothorax according to its classification. -
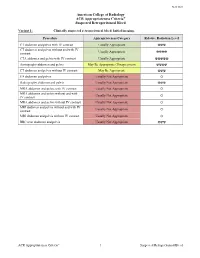
ACR Appropriateness Criteria: Suspected Retroperitoneal Bleed
New 2021 American College of Radiology ACR Appropriateness Criteria® Suspected Retroperitoneal Bleed Variant 1: Clinically suspected retroperitoneal bleed. Initial imaging. Procedure Appropriateness Category Relative Radiation Level CT abdomen and pelvis with IV contrast Usually Appropriate ☢☢☢ CT abdomen and pelvis without and with IV Usually Appropriate contrast ☢☢☢☢ CTA abdomen and pelvis with IV contrast Usually Appropriate ☢☢☢☢☢ Aortography abdomen and pelvis May Be Appropriate (Disagreement) ☢☢☢☢ CT abdomen and pelvis without IV contrast May Be Appropriate ☢☢☢ US abdomen and pelvis Usually Not Appropriate O Radiography abdomen and pelvis Usually Not Appropriate ☢☢☢ MRA abdomen and pelvis with IV contrast Usually Not Appropriate O MRA abdomen and pelvis without and with Usually Not Appropriate IV contrast O MRA abdomen and pelvis without IV contrast Usually Not Appropriate O MRI abdomen and pelvis without and with IV Usually Not Appropriate contrast O MRI abdomen and pelvis without IV contrast Usually Not Appropriate O RBC scan abdomen and pelvis Usually Not Appropriate ☢☢☢ ACR Appropriateness Criteria® 1 Suspected Retroperitoneal Bleed SUSPECTED RETROPERITONEAL BLEED Expert Panel on Vascular Imaging: Nupur Verma, MDa; Michael L. Steigner, MDb; Ayaz Aghayev, MDc; Ezana M. Azene, MD, PhDd; Suzanne T. Chong, MD, MSe; Benoit Desjardins, MD, PhDf; Riham H. El Khouli, MD, PhDg; Nicholas E. Harrison, MDh; Sandeep S. Hedgire, MDi; Sanjeeva P. Kalva, MDj; Yoo Jin Lee, MDk; David M. Mauro, MDl; Hiren J. Mehta, MDm; Mark Meissner, MDn; Anil K. Pillai, MDo; Nimarta Singh, MD, MPHp; Pal S. Suranyi, MD, PhDq; Eric E. Williamson, MDr; Karin E. Dill, MD.s Summary of Literature Review Introduction/Background Retroperitoneal bleeding is a hemorrhage into the retroperitoneal space, the space located posterior to the parietal peritoneum and anterior to the transversalis fascia.