Indigenous Organic-Oxidized Fluid Interactions in the Tissint Mars
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
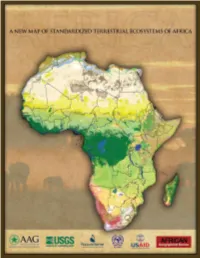
A New Map of Standardized Terrestrial Ecosystems of Africa
Major contributors to this publication include: The Association of American Geographers is a nonprofit scientific and educational society with a membership of over 10,500 individuals from more than 60 countries. AAG members are geographers and related professionals who work in the public, private, and academic sectors to advance the theory, methods, and practice of geography. This booklet is published by AAG as a special supplement to the African Geographical Review. The U.S. Geological Survey (USGS) was created in 1879 as a science agency charged with providing information and understanding to help resolve complex natural resource problems across the nation and around the world. The mission of the USGS is to provide relevant, impartial scientific information to 1) describe and understand the Earth, 2) minimize loss of life and property from natural disasters, 3) manage water, biological, energy, and mineral resources, and 4) enhance and protect our quality of life. NatureServe is an international conservation nonprofit dedicated to providing the sci- entific basis for effective conservation action. NatureServe’s network in the Americas includes more than 80 member institutions that collect and maintain a unique body of scientific knowledge about the species and ecosystems. The information products, data management tools, and biodiversity expertise that NatureServe’s scientists, technologists, and other professionals provide help meet local, national, and global conservation needs. The Regional Centre for Mapping of Resources for Development (RCMRD) was es- tablished in Nairobi, Kenya in 1975 to provide quality Geo-Information and allied Information Technology products and services in environmental and resource manage- ment for sustainable development in our member countries and beyond. -

Magnetite Biomineralization and Ancient Life on Mars Richard B Frankel* and Peter R Buseckt
Magnetite biomineralization and ancient life on Mars Richard B Frankel* and Peter R Buseckt Certain chemical and mineral features of the Martian meteorite with a mass distribution unlike terrestrial PAHs or those from ALH84001 were reported in 1996 to be probable evidence of other meteorites; thirdly, bacterium-shaped objects (BSOs) ancient life on Mars. In spite of new observations and up to several hundred nanometers long that resemble fos interpretations, the question of ancient life on Mars remains silized terrestrial microorganisms; and lastly, 10-100 nm unresolved. Putative biogenic, nanometer magnetite has now magnetite (Fe304), pyrrhotite (Fel_xS), and greigite (Fe3S4) become a leading focus in the debate. crystals. These minerals were cited as evidence because of their similarity to biogenic magnetic minerals in terrestrial Addresses magnetotactic bacteria. *Department of Physics, California Polytechnic State University, San Luis Obispo, California 93407, USA; e-mail: [email protected] The ancient life on Mars hypothesis has been extensively tDepartments of Geology and Chemistry/Biochemistry, Arizona State challenged, and alternative non-biological processes have University, Tempe, Arizona 85287-1404, USA; e-mail: [email protected] been proposed for each of the four features cited by McKay et al. [4]. In this paper we review the current situa tion regarding their proposed evidence, focusing on the Abbreviations putative biogenic magnetite crystals. BCM biologically controlled mineralization BIM biologically induced mineralization BSO bacterium-shaped object Evidence for and against ancient Martian life PAH polycyclic aromatic hydrocarbon PAHs and BSOs Reports of contamination by terrestrial organic materials [5°,6°] and the similarity of ALH84001 PAHs to non-bio genic PAHs in carbonaceous chondrites [7,8] make it Introduction difficult to positively identify PAHs of non-terrestrial, bio A 2 kg carbonaceous stony meteorite, designated genic origin. -

Lost Lake by Robert Verish
Meteorite-Times Magazine Contents by Editor Like Sign Up to see what your friends like. Featured Monthly Articles Accretion Desk by Martin Horejsi Jim’s Fragments by Jim Tobin Meteorite Market Trends by Michael Blood Bob’s Findings by Robert Verish IMCA Insights by The IMCA Team Micro Visions by John Kashuba Galactic Lore by Mike Gilmer Meteorite Calendar by Anne Black Meteorite of the Month by Michael Johnson Tektite of the Month by Editor Terms Of Use Materials contained in and linked to from this website do not necessarily reflect the views or opinions of The Meteorite Exchange, Inc., nor those of any person connected therewith. In no event shall The Meteorite Exchange, Inc. be responsible for, nor liable for, exposure to any such material in any form by any person or persons, whether written, graphic, audio or otherwise, presented on this or by any other website, web page or other cyber location linked to from this website. The Meteorite Exchange, Inc. does not endorse, edit nor hold any copyright interest in any material found on any website, web page or other cyber location linked to from this website. The Meteorite Exchange, Inc. shall not be held liable for any misinformation by any author, dealer and or seller. In no event will The Meteorite Exchange, Inc. be liable for any damages, including any loss of profits, lost savings, or any other commercial damage, including but not limited to special, consequential, or other damages arising out of this service. © Copyright 2002–2010 The Meteorite Exchange, Inc. All rights reserved. No reproduction of copyrighted material is allowed by any means without prior written permission of the copyright owner. -

Part 629 – Glossary of Landform and Geologic Terms
Title 430 – National Soil Survey Handbook Part 629 – Glossary of Landform and Geologic Terms Subpart A – General Information 629.0 Definition and Purpose This glossary provides the NCSS soil survey program, soil scientists, and natural resource specialists with landform, geologic, and related terms and their definitions to— (1) Improve soil landscape description with a standard, single source landform and geologic glossary. (2) Enhance geomorphic content and clarity of soil map unit descriptions by use of accurate, defined terms. (3) Establish consistent geomorphic term usage in soil science and the National Cooperative Soil Survey (NCSS). (4) Provide standard geomorphic definitions for databases and soil survey technical publications. (5) Train soil scientists and related professionals in soils as landscape and geomorphic entities. 629.1 Responsibilities This glossary serves as the official NCSS reference for landform, geologic, and related terms. The staff of the National Soil Survey Center, located in Lincoln, NE, is responsible for maintaining and updating this glossary. Soil Science Division staff and NCSS participants are encouraged to propose additions and changes to the glossary for use in pedon descriptions, soil map unit descriptions, and soil survey publications. The Glossary of Geology (GG, 2005) serves as a major source for many glossary terms. The American Geologic Institute (AGI) granted the USDA Natural Resources Conservation Service (formerly the Soil Conservation Service) permission (in letters dated September 11, 1985, and September 22, 1993) to use existing definitions. Sources of, and modifications to, original definitions are explained immediately below. 629.2 Definitions A. Reference Codes Sources from which definitions were taken, whole or in part, are identified by a code (e.g., GG) following each definition. -

Radar-Enabled Recovery of the Sutter's Mill Meteorite, A
RESEARCH ARTICLES the area (2). One meteorite fell at Sutter’sMill (SM), the gold discovery site that initiated the California Gold Rush. Two months after the fall, Radar-Enabled Recovery of the Sutter’s SM find numbers were assigned to the 77 me- teorites listed in table S3 (3), with a total mass of 943 g. The biggest meteorite is 205 g. Mill Meteorite, a Carbonaceous This is a tiny fraction of the pre-atmospheric mass, based on the kinetic energy derived from Chondrite Regolith Breccia infrasound records. Eyewitnesses reported hearing aloudboomfollowedbyadeeprumble.Infra- Peter Jenniskens,1,2* Marc D. Fries,3 Qing-Zhu Yin,4 Michael Zolensky,5 Alexander N. Krot,6 sound signals (table S2A) at stations I57US and 2 2 7 8 8,9 Scott A. Sandford, Derek Sears, Robert Beauford, Denton S. Ebel, Jon M. Friedrich, I56US of the International Monitoring System 6 4 4 10 Kazuhide Nagashima, Josh Wimpenny, Akane Yamakawa, Kunihiko Nishiizumi, (4), located ~770 and ~1080 km from the source, 11 12 10 13 Yasunori Hamajima, Marc W. Caffee, Kees C. Welten, Matthias Laubenstein, are consistent with stratospherically ducted ar- 14,15 14 14,15 16 Andrew M. Davis, Steven B. Simon, Philipp R. Heck, Edward D. Young, rivals (5). The combined average periods of all 17 18 18 19 20 Issaku E. Kohl, Mark H. Thiemens, Morgan H. Nunn, Takashi Mikouchi, Kenji Hagiya, phase-aligned stacked waveforms at each station 21 22 22 22 23 Kazumasa Ohsumi, Thomas A. Cahill, Jonathan A. Lawton, David Barnes, Andrew Steele, of 7.6 s correspond to a mean source energy of 24 4 24 2 25 Pierre Rochette, Kenneth L. -

A Geomorphic Classification System
A Geomorphic Classification System U.S.D.A. Forest Service Geomorphology Working Group Haskins, Donald M.1, Correll, Cynthia S.2, Foster, Richard A.3, Chatoian, John M.4, Fincher, James M.5, Strenger, Steven 6, Keys, James E. Jr.7, Maxwell, James R.8 and King, Thomas 9 February 1998 Version 1.4 1 Forest Geologist, Shasta-Trinity National Forests, Pacific Southwest Region, Redding, CA; 2 Soil Scientist, Range Staff, Washington Office, Prineville, OR; 3 Area Soil Scientist, Chatham Area, Tongass National Forest, Alaska Region, Sitka, AK; 4 Regional Geologist, Pacific Southwest Region, San Francisco, CA; 5 Integrated Resource Inventory Program Manager, Alaska Region, Juneau, AK; 6 Supervisory Soil Scientist, Southwest Region, Albuquerque, NM; 7 Interagency Liaison for Washington Office ECOMAP Group, Southern Region, Atlanta, GA; 8 Water Program Leader, Rocky Mountain Region, Golden, CO; and 9 Geology Program Manager, Washington Office, Washington, DC. A Geomorphic Classification System 1 Table of Contents Abstract .......................................................................................................................................... 5 I. INTRODUCTION................................................................................................................. 6 History of Classification Efforts in the Forest Service ............................................................... 6 History of Development .............................................................................................................. 7 Goals -

The Amino Acid Composition of the Sutterв•Žs Mill CM2 Carbonaceous
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln NASA Publications National Aeronautics and Space Administration 2014 The minoa acid composition of the Sutter’s Mill CM2 carbonaceous chondrite Aaron Burton 1NASA Johnson Space Center, [email protected] Daniel Glavin NASA Goddard Space Flight Center Jamie Elsila NASA Goddard Space Flight Center Jason Dworkin NASA Goddard Space Flight Center Peter Jenniskens SETI Institute, NASA Ames Research Center See next page for additional authors Follow this and additional works at: http://digitalcommons.unl.edu/nasapub Burton, Aaron; Glavin, Daniel; Elsila, Jamie; Dworkin, Jason; Jenniskens, Peter; and Yin, Qing-Zhu, "The minoa acid composition of the Sutter’s Mill CM2 carbonaceous chondrite" (2014). NASA Publications. 134. http://digitalcommons.unl.edu/nasapub/134 This Article is brought to you for free and open access by the National Aeronautics and Space Administration at DigitalCommons@University of Nebraska - Lincoln. It has been accepted for inclusion in NASA Publications by an authorized administrator of DigitalCommons@University of Nebraska - Lincoln. Authors Aaron Burton, Daniel Glavin, Jamie Elsila, Jason Dworkin, Peter Jenniskens, and Qing-Zhu Yin This article is available at DigitalCommons@University of Nebraska - Lincoln: http://digitalcommons.unl.edu/nasapub/134 Meteoritics & Planetary Science 1–13 (2014) doi: 10.1111/maps.12281 The amino acid composition of the Sutter’s Mill CM2 carbonaceous chondrite Aaron S. BURTON1* , Daniel P. GLAVIN2, Jamie E. ELSILA2, Jason P. DWORKIN2, Peter JENNISKENS3,4, and Qing-Zhu YIN5 1NASA Johnson Space Center, 2101 Space Center Parkway, Houston, Texas 77058, USA 2NASA Goddard Space Flight Center, 8800 Greenbelt Road, Greenbelt, Maryland 20771, USA 3SETI Institute, 189 Bernardo Avenue, Mountain View, California 94043, USA 4NASA Ames Research Center, Moffett Field, California 94035, USA 5Department of Earth and Planetary Sciences, University of California at Davis, Davis, California 95616, USA *Corresponding author. -

Mineralogy of the Sutter's Mill Carbonaceous Chondrite
44th Lunar and Planetary Science Conference (2013) 2148.pdf MINERALOGY OF THE SUTTER’S MILL CARBONACEOUS CHONDRITE. Laurence A.J. Garvie, Cen- ter for Meteorite Studies, Arizona State University, Tempe, AZ 85287-6004, USA. [email protected] Introduction: On the morning of April 22nd, 2012, The background-subtracted profiles for SM3 and 6 do a fireball was observed over Nevada and California, not show recognizable diffracted intensity for clays or with subsequent recovery of three meteorites on April amorphous materials, whereas SM8 shows weak re- 24th around Coloma and Lotus in California. These flections for clays/amorphous material (Fig. 1). pieces are significant as they are the only to be collect- XRD profiles for SM38, 41, and 65 are similar to ed before heavy rains on the 25th and 26th. To date, each other, with the majority of the diffracted intensity ~1000 g have been found as 90 small stones. The for- arising from clay/amorphous material. On top of this tuitous breakup into individual, small meteorites facili- intensity are reflections from Fe-sulfides, calcite, and tated the distribution and study of multiple individuals. magnetite. Reflections for enstatite and olivine are Initial studies show the stones to be a breccia, with weak and vary in intensity from different regions of bulk chemistry matching that of CM chondrites [1]. sampled stones and come from dispersed macroscopic The δ17O versus δ18O data of two stones [1] show one grains. The majority of the low-angle intensity is cen- (SM43) to be within the CM field, and the other tered around ~13.5Å, with a low-intensity sharp reflec- (SM51) between the CM field and Tagish Lake. -

(2000) Forging Asteroid-Meteorite Relationships Through Reflectance
Forging Asteroid-Meteorite Relationships through Reflectance Spectroscopy by Thomas H. Burbine Jr. B.S. Physics Rensselaer Polytechnic Institute, 1988 M.S. Geology and Planetary Science University of Pittsburgh, 1991 SUBMITTED TO THE DEPARTMENT OF EARTH, ATMOSPHERIC, AND PLANETARY SCIENCES IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF DOCTOR OF PHILOSOPHY IN PLANETARY SCIENCES AT THE MASSACHUSETTS INSTITUTE OF TECHNOLOGY FEBRUARY 2000 © 2000 Massachusetts Institute of Technology. All rights reserved. Signature of Author: Department of Earth, Atmospheric, and Planetary Sciences December 30, 1999 Certified by: Richard P. Binzel Professor of Earth, Atmospheric, and Planetary Sciences Thesis Supervisor Accepted by: Ronald G. Prinn MASSACHUSES INSTMUTE Professor of Earth, Atmospheric, and Planetary Sciences Department Head JA N 0 1 2000 ARCHIVES LIBRARIES I 3 Forging Asteroid-Meteorite Relationships through Reflectance Spectroscopy by Thomas H. Burbine Jr. Submitted to the Department of Earth, Atmospheric, and Planetary Sciences on December 30, 1999 in Partial Fulfillment of the Requirements for the Degree of Doctor of Philosophy in Planetary Sciences ABSTRACT Near-infrared spectra (-0.90 to ~1.65 microns) were obtained for 196 main-belt and near-Earth asteroids to determine plausible meteorite parent bodies. These spectra, when coupled with previously obtained visible data, allow for a better determination of asteroid mineralogies. Over half of the observed objects have estimated diameters less than 20 k-m. Many important results were obtained concerning the compositional structure of the asteroid belt. A number of small objects near asteroid 4 Vesta were found to have near-infrared spectra similar to the eucrite and howardite meteorites, which are believed to be derived from Vesta. -

Geologic Map of the Indian Spring Quadrangle, San Bernardino County, California
U.S. DEPARTMENT OF THE INTERIOR U.S. GEOLOGICAL SURVEY Geologic Map of the Indian Spring Quadrangle, San Bernardino County, California by H.G. Wilshire 1 Open-File Report 92-181 This report is preliminary and has not been reviewed for conformity with U.S. Geological Survey editorial standards or with the North American Stratigraphic Code. Any use of trade, product, or firm names is for descriptive purposes only and does not imply endorsement by the U.S. Government. *345 Middlefield Road MS/975, Menlo Park, CA 94025 GEOLOGIC MAP OF THE INDIAN SPRING QUADRANGLE, SAN BERNARDINO COUNTY, CALIFORNIA INTRODUCTION Eruptive rocks of the Cima volcanic field occur almost entirely within six 7.5 minute quadrangles, Indian Spring, Marl Mountains, Granite Spring, Cow Cove, Solomons Knob, and Valley Wells. The following description applies to all six quadrangles. The Cima volcanic field is a small (-300 km^) Tertiary-Quaternary alkaline basalt field in the Ivanpah highlands (Hewett, 1956), in east-central Mojave Desert (Fig. 1). The basaltic rocks, which range from late Miocene to Holocene, were erupted from at least 71 vents. The younger vents are well-formed cinder cones, whereas older vents are marked by degraded cinder cones, plugs, and crater-fill lava flows. The volcanic rocks were described in a general geologic study by Hewett (1956). The southern part of the field was mapped by Barca (1966), and fie northern part by DeWitt (1980). Topical studies of the volcanic field include the geomorphology of pediment domes (Sharp, 1957; Dohrenwend and others, 1984a,b), geomorphic and soil evolution of the lava flows (Wells and others, 1984; McFadden and others, 1984), paleontology and stratigraphy of Tertiary and Quaternary deposits of the Shadow Valley Basin (Reynolds and Nance, 1988), structural studies (Hewett, 1956; Dunne, 1977; Reynolds, 1990; Skirvin and Wells, 1990), polycyclic volcanism (Renault and Wells, 1990), and evolution of the upper mantle and lower crust beneath Cima based on xenoliths in the basalts (Wilshire, 1990; Wilshire and others, 1991). -

Critically Testing Olivine-Hosted Putative Martian Biosignatures in the Yamato 000593
1 Critically testing olivine-hosted putative Martian biosignatures in the Yamato 000593 2 meteorite - geobiological implications 3 4 Abstract: 5 On rocky planets such as Earth and Mars the serpentinization of olivine in ultramafic crust 6 produces hydrogen that can act as a potential energy source for life. Direct evidence of fluid-rock 7 interaction on Mars comes from iddingsite alteration veins found in Martian meteorites. In the 8 Yamato 000593 meteorite putative biosignatures have been reported from altered olivines in the 9 form of microtextures and associated organic material that have been compared to tubular 10 bioalteration textures found in terrestrial sub-seafloor volcanic rocks. Here we use a suite of 11 correlative, high-sensitivity, in-situ chemical and morphological analyses to characterize and re- 12 evaluate these microalteration textures in Yamato 000593, a clinopyroxenite from the shallow sub- 13 surface of Mars. We show that the altered olivine crystals have angular and micro-brecciated 14 margins and are also highly strained due to impact induced fracturing. The shape of the olivine 15 microalteration textures is in no way comparable to microtunnels of inferred biological origin 16 found in terrestrial volcanic glasses and dunites, and rather we argue that the Yamato 000593 17 microtextures are abiotic in origin. Vein filling iddingsite extends into the olivine microalteration 18 textures and contains amorphous organic carbon occurring as bands and sub-spherical 19 concentrations <300 nm across. We propose that a Martian impact event produced the micro- 20 brecciated olivine crystal margins that reacted with subsurface hydrothermal fluids to form 21 iddingsite containing organic carbon derived from abiotic sources. -

List G - Meteorites - Alphabetical List
LIST G - METEORITES - ALPHABETICAL LIST Specific name Group name Specific name Group name Abee Meteorite EH chondrites EETA 79001 Elephant Moraine Meteorites Acapulco Meteorite acapulcoite and shergottite acapulcoite stony meteorites Efremovka Meteorite CV chondrites Acfer Meteorites meteorites EH chondrites enstatite chondrites achondrites stony meteorites EL chondrites enstatite chondrites ALH 84001 Allan Hills Meteorites and Elephant Moraine meteorites achondrites Meteorites ALHA 77005 Allan Hills Meteorites and enstatite chondrites chondrites shergottite eucrite achondrites ALHA 77307 Allan Hills Meteorites and Fayetteville Meteorite H chondrites CO chondrites Frontier Mountain meteorites ALHA 81005 Allan Hills Meteorites and Meteorites achondrites Gibeon Meteorite octahedrite Allan Hills Meteorites meteorites GRA 95209 Graves Nunataks Meteorites Allende Meteorite CV chondrites and lodranite angrite achondrites Graves Nunataks meteorites Ashmore Meteorite H chondrites Meteorites Asuka Meteorites meteorites H chondrites ordinary chondrites ataxite iron meteorites Hammadah al Hamra meteorites aubrite achondrites Meteorites Barwell Meteorite L chondrites Haveroe Meteorite ureilite Baszkowka Meteorite L chondrites Haviland Meteorite H chondrites Belgica Meteorites meteorites HED meteorites achondrites Bencubbin Meteorite chondrites Hedjaz Meteorite L chondrites Bishunpur Meteorite LL chondrites Henbury Meteorite octahedrite Bjurbole Meteorite L chondrites hexahedrite iron meteorites Brenham Meteorite pallasite HL chondrites ordinary chondrites