Map Prior Authorization List Eff: 3/1/2021
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Procedure Code List Effective Jan. 1, 2020 for Preauthorization for Blue Cross and Blue Shield of New Mexico Medicare Advantage Members Only
Procedure Code List Effective Jan. 1, 2020 for Preauthorization for Blue Cross and Blue Shield of New Mexico Medicare Advantage Members only Beginning Jan. 1, 2020, providers will be required to obtain preauthorization through Blue Cross and Blue Shield of New Mexico (BCBSNM), Optum, or eviCore for certain procedures for Blue Cross Medicare Advantage members as noted in the MAPD Benefit Preauthorization Procedure Code List, Effective 1/1/2020, below. For members NOT attributed to Optum, preauthorization should be obtained from BCBSNM unless the applicable entry in the MAPD Benefit Preauthorization Procedure Code List references eviCore. For members attributed to Optum, preauthorization should be obtained from Optum, even if the applicable entry in the MAPD Benefit Preauthorization Procedure Code List references eviCore. Any entry that references eviCore should be preauthorized through eviCore except for members attributed to Optum. The member's ID Card will indicate that the member is attributed to Optum. Services performed without benefit preauthorization may be denied for payment in whole or in part, and you may not seek reimbursement from members. Member eligibility and benefits should be checked prior to every scheduled appointment. Eligibility and benefit quotes include membership status, coverage status and other important information, such as applicable copayment, coinsurance and deductible amounts. It is strongly recommended that providers ask to see the member's ID card for current information and a photo ID to guard against medical identity theft. A referral to an out-of-plan or out-of-network provider which is necessary due to network inadequacy or continuity of care must be reviewed by the BCBSNM Utilization Management or DMG (if the member is attributed to DMG this information will be reflected on the ID card) prior to a BCBSNM patient receiving care. -

Clinical Practice Guideline for Limb Salvage Or Early Amputation
Limb Salvage or Early Amputation Evidence-Based Clinical Practice Guideline Adopted by: The American Academy of Orthopaedic Surgeons Board of Directors December 6, 2019 Endorsed by: Please cite this guideline as: American Academy of Orthopaedic Surgeons. Limb Salvage or Early Amputation Evidence-Based Clinical Practice Guideline. https://www.aaos.org/globalassets/quality-and-practice-resources/dod/ lsa-cpg-final-draft-12-10-19.pdf Published December 6, 2019 View background material via the LSA CPG eAppendix Disclaimer This clinical practice guideline was developed by a physician volunteer clinical practice guideline development group based on a formal systematic review of the available scientific and clinical information and accepted approaches to treatment and/or diagnosis. This clinical practice guideline is not intended to be a fixed protocol, as some patients may require more or less treatment or different means of diagnosis. Clinical patients may not necessarily be the same as those found in a clinical trial. Patient care and treatment should always be based on a clinician’s independent medical judgment, given the individual patient’s specific clinical circumstances. Disclosure Requirement In accordance with AAOS policy, all individuals whose names appear as authors or contributors to this clinical practice guideline filed a disclosure statement as part of the submission process. All panel members provided full disclosure of potential conflicts of interest prior to voting on the recommendations contained within this clinical practice guideline. Funding Source This clinical practice guideline was funded exclusively through a research grant provided by the United States Department of Defense with no funding from outside commercial sources to support the development of this document. -

PT/OT Therapy Intake Form Required for All MSK Conditions (Including Hand) Please Use This Fax Form for NON-URGENT Requests Only
Musculoskeletal Program: PT/OT Therapy Intake Form Required for all MSK Conditions (Including Hand) Please use this fax form for NON-URGENT requests only. Failure to provide all relevant information may delay the determination. Phone and fax numbers may be found on eviCore.com under the Guidelines and Forms section. You may also log into the provider portal located on the site to submit an authorization request. URGENT (same day) REQUESTS MUST BE SUBMITTED BY PHONE Previous Reference/Auth Number (If Continued Care): Date of Submission: Place of Service: ****** Note: This is REQUIRED for WellCare Submissions ******* Service Type Requested: Physical Therapy Occupational Therapy First Name: MI: Last Name: Member ID: DOB (mm/dd/yyyy): Gender: Male Female Street Address: Apt #: City: State: Zip: PATIENT Home Phone: Cell Phone: Primary: Home Cell Member Health Plan/Insurer: First Name: Last Name: Primary Specialty: TIN: NPI: Physician Phone: Physician Fax: Address: Suite #: City: State: Zip: PROVIDER Office Contact: Ext: Email: Diagnoses: Code Description Code Description Start Date for this Request: This is a (select the most appropriate): New condition not previously treated Same/previous condition Date of initial evaluation: Date of onset of condition: Date of current findings: Primary Treatment Area: Spine: Cervical / Upper Thoracic Lower Thoracic / Lumbar / Pelvis Upper Extremity: Shoulder / Arm Elbow / Wrist / Forearm Hand Lower Extremity: Hip / Thigh Knee Ankle / Foot Other: Pelvic Pain / Incontinence Secondary Treatment Area: Spine: -
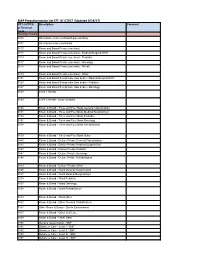
MAP Preauthorization List EFF: 8/1/2017 (Updated 8/24/17)
MAP Preauthorization List EFF: 8/1/2017 (Updated 8/24/17) CPT, HCPCS Description Comment or Revenue Code Revenue Codes 0100 All inclusive room and board plus ancillary 0101 All inclusive room and board 0110 Room and Board Private (one bed) 0111 Room and Board Private (one bed) - Medical/Surgical/GYN 0113 Room and Board Private (one bed) - Pediatric 0117 Room and Board Private (one bed) - Oncology 0118 Room and Board Private (one bed) - Rehab 0119 Room and Board Private (one bed) - Other 0121 Room and Board Semiprivate (two beds) - Medical/Surgical/GYN 0123 Room and Board Semiprivate (two beds) - Pediatric 0127 Room and Board Semiprivate (two beds) - Oncology 0128 Level 1 Rehab 0129 Level 2 Rehab - acute complex 0130 Room & Board - Three and Four Beds General Classification 0131 Room & Board - Three and Four Beds Medical/Surgical/Gyn 0133 Room & Board - Three and Four Beds Pediatric 0137 Room & Board - Three and Four Beds Oncology 0138 Room & Board - Three and Four Beds Rehabilitation 0139 Room & Board - Three and Four Beds Other 0140 Room & Board - Deluxe Private General Classification 0141 Room & Board - Deluxe Private Medical/Surgical/Gyn 0143 Room & Board - Deluxe Private Pediatric 0147 Room & Board - Deluxe Private Oncology 0148 Room & Board - Deluxe Private Rehabilitation 0149 Room & Board - Deluxe Private Other 0150 Room & Board - Ward General Classification 0151 Room & Board - Ward Medical/Surgical/Gyn 0153 Room & Board - Ward Pediatric 0157 Room & Board - Ward Oncology 0158 Room & Board - Ward Rehabilitation 0159 Room & Board - -

114.3 Cmr: Division of Health Care Finance and Policy Ambulatory Care
114.3 CMR: DIVISION OF HEALTH CARE FINANCE AND POLICY AMBULATORY CARE 114.3 CMR 40.00: RATES FOR SERVICES UNDER M.G.L. c. 152, WORKERS’ COMPENSATION ACT Section 40.01: General Provisions 40.02: General Definitions 40.03: Service and Rate Coverage Provisions 40.04: Provisions Affecting Eligible Providers 40.05: Policies for Individual Service Types 40.06: Fees 40.07: Appendices 40.08: Severability 40.01: General Provisions (1) Scope, Purpose and Effective Date. 114.3 CMR 40.00 governs the payment rates effective April 1, 2009 for purchasers of health care services under M.G.L. c. 152, the Workers’ Compensation Act. Payment rates for services provided by hospitals are set forth in 114.1 CMR 41.00. Program policies relating to medical necessity and clinical appropriateness are determined pursuant to M.G.L. c. 152 and 452 CMR 6.00. (2) Coverage. The payment rates set forth in 114.3 CMR 40.06 are full payment for services provided under M.G.L. c. 152, § 13, including any related administrative or overhead costs. The insurer, employer and health care service provider may agree upon a different payment rate for any service set forth in the fee schedule in 114.3 CMR 40.00. No employee may be held liable for the payment for health care services determined compensable under M.G.L. c. 152, § 13. (3) Administrative Bulletins. The Division may issue administrative bulletins to clarify substantive provisions of 114.3 CMR 40.00, or to publish procedure code updates and corrections. For coding updates and correction, the bulletin will list: (a) new code numbers for existing codes, with the corresponding cross references between existing and new codes numbers; (b) deleted codes for which there are no corresponding new codes; and (c) codes for entirely new services that require pricing. -

Artificial Finger Joint Replacement Due to a Giant Cell Tumor of the Tendon Sheath with Bone Destruction: a Case Report
3502 ONCOLOGY LETTERS 10: 3502-3504, 2015 Artificial finger joint replacement due to a giant cell tumor of the tendon sheath with bone destruction: A case report HUI LU, HUI SHEN, QIANG CHEN, XIANG-QIAN SHEN and SHOU-CHENG WU Department of Hand Surgery, The First Affiliated Hospital, College of Medicine, Zhejiang University, Hangzhou, Zhejiang 310003, P.R. China Received October 24, 2014; Accepted September 25, 2015 DOI: 10.3892/ol.2015.3813 Abstract. The current study presents the case of a 25-year-old excised completely and the function of the joints was recov- male who developed tumor recurrence of the proximal ered. phalange of the ring finger on the right hand 4 years after partial tumor resection surgery. An X-ray of the right hand Case report showed that the distal bone of the proximal phalange on the ring finger was destroyed. An artificial finger joint replace- A 25-year-old male was admitted to The First Affiliated ment was performed using a silicone joint for this unusual Hospital, College of Meicine, ZheJiang University (Hangzhou, tumor recurrence. The pathological findings were indicative of Zhejiang, China) with tumor recurrence of the proximal a giant cell tumor of the tendon sheath. As a result of surgery, phalange of the ring finger on the right hand 4 years after the patient's proximal interphalangeal point motion recovered partial tumor resection surgery. The patient had no history to the pre-operative level. The pre-operative and post-operative of trauma or infection and was previously healthy. A physical disabilities of the arm, at shoulder and hand and total activity examination revealed swelling of the proximal phalange of the measurement values were 1.67 and 3.33, and 255 and 243˚, finger without clear cause, multiple nodules in the local areas respectively. -

En Bloc Resection of Extra-Peritoneal Soft Tissue Neoplasms Incorporating a Type III Internal Hemipelvectomy: a Novel Approach Sanjay S Reddy1* and Norman D Bloom2
Reddy and Bloom World Journal of Surgical Oncology 2012, 10:222 http://www.wjso.com/content/10/1/222 WORLD JOURNAL OF SURGICAL ONCOLOGY REVIEW Open Access En bloc resection of extra-peritoneal soft tissue neoplasms incorporating a type III internal hemipelvectomy: a novel approach Sanjay S Reddy1* and Norman D Bloom2 Abstract Background: A type III hemipelvectomy has been utilized for the resection of tumors arising from the superior or inferior pubic rami. Methods: In eight patients, we incorporated a type III internal hemipelvectomy to achieve an en bloc R0 resection for tumors extending through the obturator foramen or into the ischiorectal fossa. The pelvic ring was reconstructed utilizing marlex mesh. This allowed for pelvic stability and abdominal wall reconstruction with obliteration of the obturator space to prevent herniations. Results: All eight patients had an R0 resection with an overall survival of 88% and with average follow up of 9.5 years. Functional evaluation utilizing the Enneking classification system, which evaluates motion, pain, stability and strength of the affected extremity, revealed a 62% excellent result and a 37% good result. No significant complications were associated with the operative procedure. Marlex mesh reconstruction provided pelvic stability and eliminated all hernial defects. Conclusion: The superior and inferior pubic rami provide a barrier to a resection for tumors that arise in the extra-peritoneal pelvis extending through the obturator foramen or ischiorectal fossa. Incorporating a type III internal -
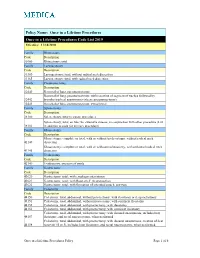
Once in a Lifetime Procedures Code List 2019 Effective: 11/14/2010
Policy Name: Once in a Lifetime Procedures Once in a Lifetime Procedures Code List 2019 Effective: 11/14/2010 Family Rhinectomy Code Description 30160 Rhinectomy; total Family Laryngectomy Code Description 31360 Laryngectomy; total, without radical neck dissection 31365 Laryngectomy; total, with radical neck dissection Family Pneumonectomy Code Description 32440 Removal of lung, pneumonectomy; Removal of lung, pneumonectomy; with resection of segment of trachea followed by 32442 broncho-tracheal anastomosis (sleeve pneumonectomy) 32445 Removal of lung, pneumonectomy; extrapleural Family Splenectomy Code Description 38100 Splenectomy; total (separate procedure) Splenectomy; total, en bloc for extensive disease, in conjunction with other procedure (List 38102 in addition to code for primary procedure) Family Glossectomy Code Description Glossectomy; complete or total, with or without tracheostomy, without radical neck 41140 dissection Glossectomy; complete or total, with or without tracheostomy, with unilateral radical neck 41145 dissection Family Uvulectomy Code Description 42140 Uvulectomy, excision of uvula Family Gastrectomy Code Description 43620 Gastrectomy, total; with esophagoenterostomy 43621 Gastrectomy, total; with Roux-en-Y reconstruction 43622 Gastrectomy, total; with formation of intestinal pouch, any type Family Colectomy Code Description 44150 Colectomy, total, abdominal, without proctectomy; with ileostomy or ileoproctostomy 44151 Colectomy, total, abdominal, without proctectomy; with continent ileostomy 44155 Colectomy, -

Use of Anterolateral Thigh Flap for Reconstruction of Traumatic Bilateral Hemipelvectomy After Major Pelvic Trauma
Al‑wageeh et al. surg case rep (2020) 6:247 https://doi.org/10.1186/s40792‑020‑01009‑2 CASE REPORT Open Access Use of anterolateral thigh fap for reconstruction of traumatic bilateral hemipelvectomy after major pelvic trauma: a case report Saleh Al‑wageeh1 , Faisal Ahmed2* , Khalil Al‑naggar3 , Mohammad Reza Askarpour4 and Ebrahim Al‑shami5 Abstract Background: Major pelvic trauma (MPT) with traumatic hemipelvectomy (THP) is rare, but it is a catastrophic health problem caused by high‑energy injury leading to separation of the lower extremity from the axial skeleton, which is associated with a high incidence of intra‑abdominal and multi‑systemic injuries. THP is generally performed as a lifesaving protocol to return the patient to an active life. Case report: A 12‑year male patient exposed to major pelvic trauma with bilateral THP survived the trauma and mul‑ tiple lifesaving operations. The anterolateral thigh fap is the method used for wound reconstruction. The follow‑up was ended with colostomy and cystostomy with wheelchair mobilization. To the best of our knowledge, there have been a few bilateral THP reports, and our case is the second one to be successfully treated with an anterolateral thigh fap. Conclusion: MPT with THP is the primary cause of death among trauma patients. Life‑threatening hemorrhage is the usual cause of death, which is a strong indication for THP to save life. Keywords: Amputation, Hemipelvectomy, Myocutaneous fap, Reconstruction, Trauma Introduction A few victims survive these injuries, and the actual Major pelvic trauma (MPT) associated with traumatic incidence is unknown, but it is usually underestimated hemipelvectomy (THP) was described frst by Turnbull in [3]. -

MAP Prior Authorization List EFF: 11/01/2017 (Updated 11/7/2017)
MAP Prior Authorization List EFF: 11/01/2017 (Updated 11/7/2017) CPT, HCPCS Description Comment or Revenue Code Inpatient All Inpatient admissions require authorization Revenue Codes 0100 All inclusive room and board plus ancillary 0101 All inclusive room and board 0110 Room and Board Private (one bed) 0111 Room and Board Private (one bed) - Medical/Surgical/GYN 0113 Room and Board Private (one bed) - Pediatric 0117 Room and Board Private (one bed) - Oncology 0119 Room and Board Private (one bed) - Other 0121 Room and Board Semiprivate (two beds) - Medical/Surgical/GYN 0123 Room and Board Semiprivate (two beds) - Pediatric 0127 Room and Board Semiprivate (two beds) - Oncology 0130 Room & Board - Three and Four Beds General Classification 0131 Room & Board - Three and Four Beds Medical/Surgical/Gyn 0133 Room & Board - Three and Four Beds Pediatric 0137 Room & Board - Three and Four Beds Oncology 0139 Room & Board - Three and Four Beds Other 0140 Room & Board - Deluxe Private General Classification 0141 Room & Board - Deluxe Private Medical/Surgical/Gyn 0143 Room & Board - Deluxe Private Pediatric 0147 Room & Board - Deluxe Private Oncology 0149 Room & Board - Deluxe Private Other 0150 Room & Board - Ward General Classification 0151 Room & Board - Ward Medical/Surgical/Gyn 0153 Room & Board - Ward Pediatric 0157 Room & Board - Ward Oncology 0159 Room & Board - Ward Other 0160 Room & Board - Other General Classification 0164 Other Room & Board - Sterile Environment 0167 Room & Board - Other Self Care 0169 Room & Board - Other Other 0190 General -

184591/2021/Estt-Ne Hr
168 184591/2021/ESTT-NE_HR PUSHPAWATI SINGHANIA HOSPITAL & RESEARCH INSTITUTE A MULTISPECIALITY HOSPITAL (formally known as Puspawati Singhania Research Institute for liver,Renal & Digestive Diseases) (w.e.f. 01.04.2020) OPD CHARGES FEES (Rs) SR. NO. CONSULTANT NAME FIRST SUBS. GASTROENTEROLOGY 1PROF.(DR). R.K.TANDON 20001500 2LT. COL. (DR) ARUN KUMAR 12001200 3DR. DINESH SINGHAL 10001000 4DR. MANOJ KUMAR 10001000 5DR. NRIPEN SAIKIA 10001000 6DR. RAJIV BAIJAL 10001000 7DR. RAHUL GUPTA 20002000 8DR. SHUBHAM VATSYA 900900 SURGICAL GASTROENTEROLOGY & LIVER TRANSPLANT 1DR. K.R VASUDEVAN 12001200 2DR. MANOJ GUPTA 10001000 3DR. BHUSHAN P. BHOLA 12001200 NEPHROLOGY 1DR. SANJEEV SAXENA 12001200 2DR. RAVI BANSAL 10001000 3DR. RAJESH GOEL 800800 UROLOGY 1DR. P.P.SINGH 12001200 2DR. JAGDEEP BALYAN 11001100 3DR. A.S.MALHOTRA 11001100 CARDIOLOGIST 1DR. T.S.KLER 10001000 2DR. VINAYAK AGRAWAL 12001200 3DR. AVINASH VERMA 10001000 4DR. S. N. PATHAK 10001000 5DR. ABHINAV AGGARWAL 10001000 CARDIO SURGERY 1DR. BALRAM AIRAN 10001000 2DR. VIJAY MOHAN KOHLI 12501250 3DR GAURAV GUPTA 10001000 NEUROLOGOY 1DR. SHAMSHER DWIVEDEE 20001800 NEURO SURGERY 1DR. SUMIT GOYAL 10001000 ENDOCRINOLOGY 1DR. MONIKA SHARMA 1001000 PULMONOLOGY 1DR. G.C. KHILNANI 15001500 2DR. NEETU JAIN 10001000 G.I SURGERY 1DR. SANJAY CHAUREY 15001500 2DR. RAJEEV KHANNA 12001000 3DR. HARISH KAPILA 15001500 ONCOLOGY (SURGERY) 1DR. ARVIND KUMAR 15001500 2DR. SALEEM NAYAK 15001500 3DR. VIVEK GUPTA 15001500 4DR. VIKRANT SHARMA 15001500 ONCOLOGY 1DR. AMISH VORA 15001500 2DR. AMIT UPADHYAY 15001500 ORTHOPEDICS 1DR. P.P. KOTWAL 15001500 2DR. GAURAV PRAKASH BHARDWAJ 12001200 Page 1 169 184591/2021/ESTT-NE_HR 3DR. U.K.SADHOO 12001200 4DR. G.S.TUCKER 12001200 5DR. ANIL MISHRA 10001000 6DR. -
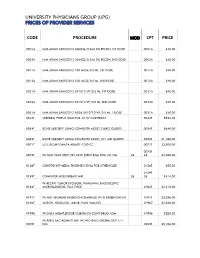
Code Procedure Cpt Price University Physicians Group
UNIVERSITY PHYSICIANS GROUP (UPG) PRICES OF PROVIDER SERVICES CODE PROCEDURE MOD CPT PRICE 0001A IMM ADMN SARSCOV2 30MCG/0.3ML DIL RECON 1ST DOSE 0001A $40.00 0002A IMM ADMN SARSCOV2 30MCG/0.3ML DIL RECON 2ND DOSE 0002A $40.00 0011A IMM ADMN SARSCOV2 100 MCG/0.5 ML 1ST DOSE 0011A $40.00 0012A IMM ADMN SARSCOV2 100 MCG/0.5 ML 2ND DOSE 0012A $40.00 0021A IMM ADMN SARSCOV2 5X1010 VP/0.5 ML 1ST DOSE 0021A $40.00 0022A IMM ADMN SARSCOV2 5X1010 VP/0.5 ML 2ND DOSE 0022A $40.00 0031A IMM ADMN SARSCOV2 AD26 5X10^10 VP/0.5 ML 1 DOSE 0031A $40.00 0042T CEREBRAL PERFUS ANALYSIS, CT W/CONTRAST 0042T $954.00 0054T BONE SURGERY USING COMPUTER ASSIST, FLURO GUIDED 0054T $640.00 0055T BONE SURGERY USING COMPUTER ASSIST, CT/ MRI GUIDED 0055T $1,188.00 0071T U/S LEIOMYOMATA ABLATE <200 CC 0071T $2,500.00 0075T 0075T PR TCAT PLMT XTRC VRT CRTD STENT RS&I PRQ 1ST VSL 26 26 $2,208.00 0126T CAROTID INT-MEDIA THICKNESS EVAL FOR ATHERSCLER 0126T $55.00 0159T 0159T COMPUTER AIDED BREAST MRI 26 26 $314.00 PR RECTAL TUMOR EXCISION, TRANSANAL ENDOSCOPIC 0184T MICROSURGICAL, FULL THICK 0184T $2,315.00 0191T PR ANT SEGMENT INSERTION DRAINAGE W/O RESERVOIR INT 0191T $2,396.00 01967 ANESTH, NEURAXIAL LABOR, PLAN VAG DEL 01967 $2,500.00 01996 PR DAILY MGMT,EPIDUR/SUBARACH CONT DRUG ADM 01996 $285.00 PR PERQ SAC AGMNTJ UNI W/WO BALO/MCHNL DEV 1/> 0200T NDL 0200T $5,106.00 PR PERQ SAC AGMNTJ BI W/WO BALO/MCHNL DEV 2/> 0201T NDLS 0201T $9,446.00 PR INJECT PLATELET RICH PLASMA W/IMG 0232T HARVEST/PREPARATOIN 0232T $1,509.00 0234T PR TRANSLUMINAL PERIPHERAL ATHERECTOMY, RENAL