Pulsar-18 US Rev C 18.05.2017 11:49 Seite 1
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Chapter 16 the Sun and Stars
Chapter 16 The Sun and Stars Stargazing is an awe-inspiring way to enjoy the night sky, but humans can learn only so much about stars from our position on Earth. The Hubble Space Telescope is a school-bus-size telescope that orbits Earth every 97 minutes at an altitude of 353 miles and a speed of about 17,500 miles per hour. The Hubble Space Telescope (HST) transmits images and data from space to computers on Earth. In fact, HST sends enough data back to Earth each week to fill 3,600 feet of books on a shelf. Scientists store the data on special disks. In January 2006, HST captured images of the Orion Nebula, a huge area where stars are being formed. HST’s detailed images revealed over 3,000 stars that were never seen before. Information from the Hubble will help scientists understand more about how stars form. In this chapter, you will learn all about the star of our solar system, the sun, and about the characteristics of other stars. 1. Why do stars shine? 2. What kinds of stars are there? 3. How are stars formed, and do any other stars have planets? 16.1 The Sun and the Stars What are stars? Where did they come from? How long do they last? During most of the star - an enormous hot ball of gas day, we see only one star, the sun, which is 150 million kilometers away. On a clear held together by gravity which night, about 6,000 stars can be seen without a telescope. -

Surfing on a Flash of Light from an Exploding Star ______By Abraham Loeb on December 26, 2019
Surfing on a Flash of Light from an Exploding Star _______ By Abraham Loeb on December 26, 2019 A common sight on the beaches of Hawaii is a crowd of surfers taking advantage of a powerful ocean wave to reach a high speed. Could extraterrestrial civilizations have similar aspirations for sailing on a powerful flash of light from an exploding star? A light sail weighing less than half a gram per square meter can reach the speed of light even if it is separated from the exploding star by a hundred times the distance of the Earth from the Sun. This results from the typical luminosity of a supernova, which is equivalent to a billion suns shining for a month. The Sun itself is barely capable of accelerating an optimally designed sail to just a thousandth of the speed of light, even if the sail starts its journey as close as ten times the Solar radius – the closest approach of the Parker Solar Probe. The terminal speed scales as the square root of the ratio between the star’s luminosity over the initial distance, and can reach a tenth of the speed of light for the most luminous stars. Powerful lasers can also push light sails much better than the Sun. The Breakthrough Starshot project aims to reach several tenths of the speed of light by pushing a lightweight sail for a few minutes with a laser beam that is ten million times brighter than sunlight on Earth (with ten gigawatt per square meter). Achieving this goal requires a major investment in building the infrastructure needed to produce and collimate the light beam. -

Sludgefinder 2 Sixth Edition Rev 1
SludgeFinder 2 Instruction Manual 2 PULSAR MEASUREMENT SludgeFinder 2 (SIXTH EDITION REV 1) February 2021 Part Number M-920-0-006-1P COPYRIGHT © Pulsar Measurement, 2009 -21. All rights reserved. No part of this publication may be reproduced, transmitted, transcribed, stored in a retrieval system, or translated into any language in any form without the written permission of Pulsar Process Measurement Limited. WARRANTY AND LIABILITY Pulsar Measurement guarantee for a period of 2 years from the date of delivery that it will either exchange or repair any part of this product returned to Pulsar Process Measurement Limited if it is found to be defective in material or workmanship, subject to the defect not being due to unfair wear and tear, misuse, modification or alteration, accident, misapplication, or negligence. Note: For a VT10 or ST10 transducer the period of time is 1 year from date of delivery. DISCLAIMER Pulsar Measurement neither gives nor implies any process guarantee for this product and shall have no liability in respect of any loss, injury or damage whatsoever arising out of the application or use of any product or circuit described herein. Every effort has been made to ensure accuracy of this documentation, but Pulsar Measurement cannot be held liable for any errors. Pulsar Measurement operates a policy of constant development and improvement and reserves the right to amend technical details, as necessary. The SludgeFinder 2 shown on the cover of this manual is used for illustrative purposes only and may not be representative -

Pulsar's PVT Measures Astronaut's Behavioral Alertness While on The
Pulsar’s PVT measures astronaut’s behavioral alertness while on the ISS Dec 2012 R&D Case Studies Challenges The demands placed on an astronaut—who must both subsist in an artificial environment and carry out mission objectives—require that he or she operate at peak alertness. Long-distant verbal check- ins with medical personnel are not sufficient to provide timely, quantitative measurements of an astronaut’s vigilance. Pulsar’s president and CEO Daniel Mollicone trained with Dinges at the university en route to earning his doctorate in Improve the overall health biomedical engineering. In 2006 Dinges asked Mollicone, who and performance of our astronauts. had worked on NASA projects in the past, if the company could develop the software for ISS. Products and services Solution PVT For astronauts working aboard the International Space Station (ISS) in low-Earth orbit, getting adequate sleep is a challenge. For one, there’s that demanding and often unpredictable schedule. Maybe there’s an experiment needing attention one minute, a vehicle docking the next, followed by unexpected station repairs that need immediate attention. Next among sleep inhibitors is the catalogue of microgravityrelated ailments, such as aching joints and backs, motion sickness, and uncomfortable sleeping positions. “When people get high And then there is the body’s thrown-off perception of time. Because on the ISS the Sun rises and sets every 45 fatigue scores on the minutes, the body’s circadian rhythm—the internal clock that, among its functions, regulates the sleep cycle PVT they always say, based on Earth’s 24-hour rotation—falls out of sync. -

Exploring Solar Energy Student Guide
U.S. DEPARTMENT OF Energy Efficiency & ENERGY EDUCATION AND WORKFORCE DEVELOPMENT ENERGY Renewable Energy Exploring Solar Energy Student Guide (Seven Activities) Grades: 5-8 Topic: Solar Owner: NEED This educational material is brought to you by the U.S. Department of Energy’s Office of Energy Efficiency and Renewable Energy. Name: fXPLORING SOLAR fNfRGY student Guide WHAT IS SOLAR ENERGY? Every day, the sun radiates (sends out) an enormous amount of energy. It radiates more energy in one second than the world has used since time began. This energy comes from within the sun itself. Like most stars, the sun is a big gas ball made up mostly of hydrogen and helium atoms. The sun makes energy in its inner core in a process called nuclear fusion. During nuclear fusion, the high pressure and temperature in the sun's core cause hydrogen (H) atoms to come apart. Four hydrogen nuclei (the centers of the atoms) combine, or fuse, to form one helium atom. During the fusion process, radiant energy is produced. It takes millions of years for the radiant energy in the sun's core to make its way to the solar surface, and then ust a little over eight minutes to travel the 93 million miles to earth. The radiant energy travels to the earth at a speed of 186,000 miles per second, the speed of light. Only a small portion of the energy radiated by the sun into space strikes the earth, one part in two billion. Yet this amount of energy is enormous. Every day enough energy strikes the United States to supply the nation's energy needs for one and a half years. -

LESSON 4, STARS Objectives
Chapter 8, Astronomy LESSON 4, STARS Objectives . Define some of the properties of stars. Compare the evolutionary paths of star types. Main Idea . Stars vary in their size, their brightness, and their distance from Earth. Vocabulary . star a large, hot ball of gases, which is held together by gravity and gives off its own light . constellation a group of stars that appears to form a pattern . parallax the apparent shift in an objects position when viewed from two locations. light-year . nebula . supernova . black hole What are stars? . A star is a large, hot ball of gases, held together by gravity, that gives off its own light. A constellation is a group of stars that appear to form a pattern. As Earth revolves, different constellations can be seen, like Orion, which is a winter constellation in the Northern Hemisphere. Constellations are classified by the seasons they appear in. Finding the Big Dipper in Ursa Major, the Great Bear, can help you find Polaris, the North Star. If you are unsure of directions, the North Star can help you. Because of our perspective, the stars in the sky form pictures, as we look at them from Earth. If we looked at the same stars from outside our solar system, the pictures would not look the same. Finding the Distance to a Star . Viewed from different points in Earth’s orbit, some stars seem to change position slightly compared to stars farther away. The apparent shift in an objects position when viewed from two locations is called parallax. Astronomers use parallax to find the distance of a star from Earth. -

UNDERSTANDING SPACE WEATHER Part III: the Sun’S Domain
UNDERSTANDING SPACE WEATHER Part III: The Sun’s Domain KEITH STRONG, NICHOLEEN VIALL, JOAN SCHMELZ, AND JULIA SABA The solar wind is a continuous, varying outflow of high-temperature plasma, traveling at hundreds of kilometers per second and stretching to over 100 au from the Sun. his paper is the third in a series of five papers Our intention is to provide a basic context so that about understanding the phenomena that cause they can produce more informative and interesting T space weather and their impacts. These papers program content with respect to space weather. are a primer for meteorological and climatological Strong et al. (2012, hereafter Part I) described the students who may be interested in the outside forces production of solar energy and its tortuous journey that directly or indirectly affect Earth’s neutral at- from the solar core to the surface. That paper dealt mosphere. The series is also designed to provide with long-term variations of the solar output. Strong background information for the many media weather et al. (2017, hereafter Part II) addressed issues related forecasters who often refer to solar-related phenom- to short-term solar variability (primarily flares and ena such as flares, coronal mass ejections (CMEs), CMEs). This paper (Part III) deals with the variations coronal holes, and geomagnetic storms. Often those in the outflow of particles from the Sun throughout the presentations are misleading or definitively wrong. solar system to its outer boundaries: the Sun’s domain (see Fig. 1). The forthcoming Part IV (“The Sun–Earth Connection”) will deal with how the variable solar AFFILIATIONS: STRONG,* VIALL, AND SABA*—NASA Goddard radiation, magnetic field, and particle outputs inter- Space Flight Center, Greenbelt, Maryland; SCHMELZ—Universities act with Earth’s magnetic field and atmosphere. -

Chapter 15--Our
2396_AWL_Bennett_Ch15/pt5 6/25/03 3:40 PM Page 495 PART V STELLAR ALCHEMY 2396_AWL_Bennett_Ch15/pt5 6/25/03 3:40 PM Page 496 15 Our Star LEARNING GOALS 15.1 Why Does the Sun Shine? • Why was the Sun dimmer in the distant past? • What process creates energy in the Sun? • How do we know what is happening inside the Sun? • Why does the Sun’s size remain stable? • What is the solar neutrino problem? Is it solved ? • How did the Sun become hot enough for fusion 15.4 From Core to Corona in the first place? • How long ago did fusion generate the energy we 15.2 Plunging to the Center of the Sun: now receive as sunlight? An Imaginary Journey • How are sunspots, prominences, and flares related • What are the major layers of the Sun, from the to magnetic fields? center out? • What is surprising about the temperature of the • What do we mean by the “surface” of the Sun? chromosphere and corona, and how do we explain it? • What is the Sun made of? 15.5 Solar Weather and Climate 15.3 The Cosmic Crucible • What is the sunspot cycle? • Why does fusion occur in the Sun’s core? • What effect does solar activity have on Earth and • Why is energy produced in the Sun at such its inhabitants? a steady rate? 496 2396_AWL_Bennett_Ch15/pt5 6/25/03 3:40 PM Page 497 I say Live, Live, because of the Sun, from some type of chemical burning similar to the burning The dream, the excitable gift. -

The Sun As a Guide to Stellar Physics This Page Intentionally Left Blank the Sun As a Guide to Stellar Physics
The Sun as a Guide to Stellar Physics This page intentionally left blank The Sun as a Guide to Stellar Physics Edited by Oddbjørn Engvold Professor emeritus, Rosseland Centre for Solar Physics, Institute of Theoretical Astrophysics, University of Oslo, Oslo, Norway Jean-Claude Vial Emeritus Senior Scientist, Institut d’Astrophysique Spatiale, CNRS-Universite´ Paris-Sud, Orsay, France Andrew Skumanich Emeritus Senior Scientist, High Altitude Observatory, National Center for Atmospheric Research, Boulder, Colorado, United States Elsevier Radarweg 29, PO Box 211, 1000 AE Amsterdam, Netherlands The Boulevard, Langford Lane, Kidlington, Oxford OX5 1GB, United Kingdom 50 Hampshire Street, 5th Floor, Cambridge, MA 02139, United States Copyright © 2019 Elsevier Inc. All rights reserved. No part of this publication may be reproduced or transmitted in any form or by any means, electronic or mechanical, including photocopying, recording, or any information storage and retrieval system, without permission in writing from the publisher. Details on how to seek permission, further information about the Publisher’s permissions policies and our arrangements with organizations such as the Copyright Clearance Center and the Copyright Licensing Agency, can be found at our website: www.elsevier.com/permissions. This book and the individual contributions contained in it are protected under copyright by the Publisher (other than as may be noted herein). Notices Knowledge and best practice in this field are constantly changing. As new research and experience broaden our understanding, changes in research methods, professional practices, or medical treatment may become necessary. Practitioners and researchers must always rely on their own experience and knowledge in evaluating and using any information, methods, compounds, or experiments described herein. -

Analyzing an Earth-Sun Model to Explain the Stars Seen in The
EXPLORATION 1 Analyzing an Earth-Sun Model to Explain the Stars Seen in the Sky A group of stars that form a pattern is called a constellation. The constellation Ursa Major also has an inner pattern known as the Big Dipper. But if you look for the Big Dipper, you will see that it is not always in the same position in the night sky. In addition to the movement of the stars across the sky every night, their rising times change throughout the year. So the constellations visible in the early evening are different in winter, spring, summer, and fall. 3. Discuss When you look at the night sky, what do you see? The Big Dipper is one of the most familiar star patterns in the northern sky. It looks Changes in Stars Seen in the Night Sky like a ladle used to scoop water. Just as the sun appears to move in a path across the sky, stars in the night sky also appear to move. They seem to change locations nightly. 4. Why do the stars appear to move across the sky every night? A. Because Earth orbits the sun once a year. B. Because Earth tilts on its axis. C. Because Earth rotates once every 24 hours. 5. Act With your class, investigate why star patterns change yearly. • Several students form a large circle. They represent the stars in the night sky. One student represents the sun and stands in the center. One student represents Earth and stands between the stars and the sun. The head of the student who is Earth represents the North Pole of Earth. -
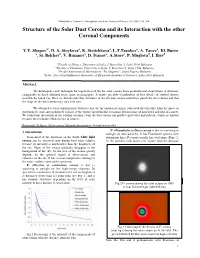
Structure of the Solar Dust Corona and Its Interaction with the Other Coronal Components
Published in: Journal of Atmospheric and Solar-Terrestrial Physics 70 (2008) 356–364 Structure of the Solar Dust Corona and its Interaction with the other Coronal Components Y.Y. Shopov a* , D. A. Stoykova a, K. Stoitchkova a, L.T.Tsankov a, A. Tanev a, Kl. Burin a, St. Belchev b, V. Rusanov a, D. Ivanov a, A.Stoev c, P. Muglova d, I. Iliev d aFaculty of Physics, University of Sofia, J. Bourchier 5, Sofia 1164, Bulgaria bFaculty of Chemistry, University of Sofia, J. Bourchier 1, Sofia 1164, Bulgaria cPeople Astronomical Observatory “Yu. Gagarin”, Stara Zagora, Bulgaria dSolar- Terrestrial Influences laboratory of Bulgarian Academy of Sciences, Sofia 1000, Bulgaria Abstract. We developed a new technique for registration of the far solar corona from ground based observations at distances comparable to those obtained from space coronagraphs. It makes possible visualization of fine details of studied objects invisible by naked eye. Here we demonstrate that streamers of the electron corona sometimes punch the dust corona and that the shape of the dust corona may vary with time. We obtained several experimental evidences that the far coronal streamers (observed directly only from the space or stratosphere) emit only in discrete regions of the visible spectrum like resonance fluorescence of molecules and ions in comets. We found that interaction of the coronal streamers with the dust corona can produce molecules and radicals, which are known to cause the resonance fluorescence in comets. Keywords: Eclipses, Solar corona, Infrared observations, Interplanetary dust F- (Fraunhofer or Dust) corona is due to scattering of 1. Introduction sunlight on dust particles. -
Spectral Classification of Stars
29:50 Astronomy Lab #6 Stars, Galaxies, and the Universe Name Partner(s) Date Grade Category Max Points Points Received On Time 5 Printed Copy 5 Lab Work 90 Total 100 Spectral Classification of Stars 1. Introduction Scientists across all fields use classification systems to help them sub-divide the vast universe of objects and phenomena into smaller groups, making them easier to study. In biology, life can be categorized by cellular properties. Animals are separated from plants, cats separated from dogs, oak trees separated from pine trees, etc. This framework allows biologists to better understand how processes are FIGURE 1: SPECTRUM OF VEGA interconnected and quickly predict characteristics of newly discovered species. In Astronomy, the stellar classification system allows scientists to understand the p r o p e r t i e s o f s t a r s . E a r l y astronomers began to realize that the extensive number of stars exhibited patterns related to the starʼs color and temperature. This classification was good, but had limitations (especially for studying distant stars). By the early 1900ʼs, a classification system based on the observed stellar spectra was developed and is still used today. A STELLAR SPECTRUM is a measurement of a 1 Spectral Classification of Stars starʼs brightness across of range of wavelengths (or frequencies). It is in a sense a “fingerprint” for the star, containing features that reveal the chemical composition, age, and temperature. This measurement is made by “breaking up” the light from the star into individual wavelengths, much like how a prism or a raindrop separates the sunlight into a rainbow.