Phylogenetic Relationships of the Soft-Shelled Turtles (Family Trionychidae)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A New Xinjiangchelyid Turtle from the Middle Jurassic of Xinjiang, China and the Evolution of the Basipterygoid Process in Mesozoic Turtles Rabi Et Al
A new xinjiangchelyid turtle from the Middle Jurassic of Xinjiang, China and the evolution of the basipterygoid process in Mesozoic turtles Rabi et al. Rabi et al. BMC Evolutionary Biology 2013, 13:203 http://www.biomedcentral.com/1471-2148/13/203 Rabi et al. BMC Evolutionary Biology 2013, 13:203 http://www.biomedcentral.com/1471-2148/13/203 RESEARCH ARTICLE Open Access A new xinjiangchelyid turtle from the Middle Jurassic of Xinjiang, China and the evolution of the basipterygoid process in Mesozoic turtles Márton Rabi1,2*, Chang-Fu Zhou3, Oliver Wings4, Sun Ge3 and Walter G Joyce1,5 Abstract Background: Most turtles from the Middle and Late Jurassic of Asia are referred to the newly defined clade Xinjiangchelyidae, a group of mostly shell-based, generalized, small to mid-sized aquatic froms that are widely considered to represent the stem lineage of Cryptodira. Xinjiangchelyids provide us with great insights into the plesiomorphic anatomy of crown-cryptodires, the most diverse group of living turtles, and they are particularly relevant for understanding the origin and early divergence of the primary clades of extant turtles. Results: Exceptionally complete new xinjiangchelyid material from the ?Qigu Formation of the Turpan Basin (Xinjiang Autonomous Province, China) provides new insights into the anatomy of this group and is assigned to Xinjiangchelys wusu n. sp. A phylogenetic analysis places Xinjiangchelys wusu n. sp. in a monophyletic polytomy with other xinjiangchelyids, including Xinjiangchelys junggarensis, X. radiplicatoides, X. levensis and X. latiens. However, the analysis supports the unorthodox, though tentative placement of xinjiangchelyids and sinemydids outside of crown-group Testudines. A particularly interesting new observation is that the skull of this xinjiangchelyid retains such primitive features as a reduced interpterygoid vacuity and basipterygoid processes. -

Zur Ökologie Von Cycloderma Aubryi (Dumeril, 1856) in Gabun
Zur Ökologie von Cycloderma aubryi (DuMERIL, 1856) in Gabun DIETER GRAMENTZ Abstract On the ecology of Cycloderma aubryi (DuMERTL, 1856) in Gabon. Subadult and adult turtles preferably inhabit areas with reed and bays with emersed vegetation. However, they avoid these areas when the water level is below 100 cm. Juvenile turtles inhabit temporarily inundated areas in the forest. The turtles bury themselves into the soil when their water habitats dry out. The average water depth in reed areas is 127 cm, in bays of land spits 135 cm, andin the forest 50 cm. Of 51 turtles examined, only one individual had a bite mark on one femoral flap caused by another softshell turtle. The pH-value varies from 5.0 in the forest to 6.0 in the other habitats. Eggs are laid in the minor dry season from December to January. The turtles feed on fish. The average body temperature of the turtles was 30,0 °C. The body temperatures were always above water temperature. The lowest average water tem perature was measured in the forest and the highest in the bays. Endoparasitic tapeworms were found in the intestines, a nematode in the body cavity, and leeches may occur on practically all parts of the body. Key words: Testudines: Trionychidae: Cyclanorbinae: Cycloderma aubryi; distribution; habitat; movements; diet; competition; predators; parasites; body temperature; physical and chemical data of the environment. Zusammenfassung Subadulte und adulte Schildkröten bewohnen bevorzugt Schilfgebiete und Gewässereinbuch tungen mit emerser Vegetation, in denen sie aber nicht mehr vorkommen, wenn die Wassertiefe unter lOO cm sinkt. Juvenile Schildkröten bewohnen temporär überschwemmte Waldgebiete. -

Zur Morphologie Und Merkmalsvariation Von Cycloderma Aubryi (DUMERIL, 1856)
Zur Morphologie und Merkmalsvariation von Cycloderma aubryi (DUMERIL, 1856) DIETER GR AMENTZ Abstract On the morphology and the variation of the pattern l!f Cycloderma aubryi ( DuMERIL, 1856). The relationships of carapace width, plastron length, lip width, tail flap length, femoral flap width, tail length, and mass to carapace length were calculated. The carapace stretches during growth in relation to the carapace width. Males have longer tails than females and in mature males, it reaches always beyond the edge of the carapace. Females grow !arger than males. The colouration of the turtles changes considerably during ontogeny and practically all parts of the body are affected. The tuberculation on the dorsal side of juvenils disappears with increasing age. The number of antebrachial scutes on the fore legs can be symmetrical or asymmetrical and varies from 5-8. The species presumably matures at a carapace length of 30-32 cm. Key words: Testudines: Trionychidae: Cyclanorbinae: Cycloderma aubryi; sexual dimorphism, colouration, external characteristics, maturity. Zusammenfassung Es wurden die Verhältnisse der Carapaxbreite, Plastronlänge, Lippenbreite, Schwanz klappenlänge, Femoralklappenbreite, Schwanzlänge und Masse zur Carapaxlänge berech net. Die Carapaxlänge streckt sich während des Wachstums im Verhältnis zur Carapaxbreite. Die Männchen haben längere Schwänze als die Weibchen. Der Schwanz reicht bei erwachsenen Männchen immer über den Carapaxrand hinaus. Die Weibchen werden größer als die Männchen. Die Färbung der Schildkröten verändert sich sehr stark während der Ontogenese; es si nd praktisch alle Körperteile davon betroffen. Die Tuberkulation auf der Oberseite der Jungtiere verschwindet mit fortschreitendem Alter. Die Anzahl der Ante brachialschuppen auf den Vorderbeinen kann symmetrisch oder unsymmetrisch vorliegen und variiert vo n 5-8. -
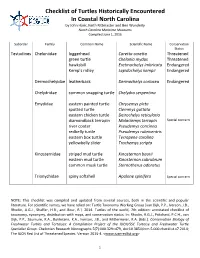
N.C. Turtles Checklist
Checklist of Turtles Historically Encountered In Coastal North Carolina by John Hairr, Keith Rittmaster and Ben Wunderly North Carolina Maritime Museums Compiled June 1, 2016 Suborder Family Common Name Scientific Name Conservation Status Testudines Cheloniidae loggerhead Caretta caretta Threatened green turtle Chelonia mydas Threatened hawksbill Eretmochelys imbricata Endangered Kemp’s ridley Lepidochelys kempii Endangered Dermochelyidae leatherback Dermochelys coriacea Endangered Chelydridae common snapping turtle Chelydra serpentina Emydidae eastern painted turtle Chrysemys picta spotted turtle Clemmys guttata eastern chicken turtle Deirochelys reticularia diamondback terrapin Malaclemys terrapin Special concern river cooter Pseudemys concinna redbelly turtle Pseudemys rubriventris eastern box turtle Terrapene carolina yellowbelly slider Trachemys scripta Kinosternidae striped mud turtle Kinosternon baurii eastern mud turtle Kinosternon subrubrum common musk turtle Sternotherus odoratus Trionychidae spiny softshell Apalone spinifera Special concern NOTE: This checklist was compiled and updated from several sources, both in the scientific and popular literature. For scientific names, we have relied on: Turtle Taxonomy Working Group [van Dijk, P.P., Iverson, J.B., Rhodin, A.G.J., Shaffer, H.B., and Bour, R.]. 2014. Turtles of the world, 7th edition: annotated checklist of taxonomy, synonymy, distribution with maps, and conservation status. In: Rhodin, A.G.J., Pritchard, P.C.H., van Dijk, P.P., Saumure, R.A., Buhlmann, K.A., Iverson, J.B., and Mittermeier, R.A. (Eds.). Conservation Biology of Freshwater Turtles and Tortoises: A Compilation Project of the IUCN/SSC Tortoise and Freshwater Turtle Specialist Group. Chelonian Research Monographs 5(7):000.329–479, doi:10.3854/crm.5.000.checklist.v7.2014; The IUCN Red List of Threatened Species. -

A Phylogenomic Analysis of Turtles ⇑ Nicholas G
Molecular Phylogenetics and Evolution 83 (2015) 250–257 Contents lists available at ScienceDirect Molecular Phylogenetics and Evolution journal homepage: www.elsevier.com/locate/ympev A phylogenomic analysis of turtles ⇑ Nicholas G. Crawford a,b,1, James F. Parham c, ,1, Anna B. Sellas a, Brant C. Faircloth d, Travis C. Glenn e, Theodore J. Papenfuss f, James B. Henderson a, Madison H. Hansen a,g, W. Brian Simison a a Center for Comparative Genomics, California Academy of Sciences, 55 Music Concourse Drive, San Francisco, CA 94118, USA b Department of Genetics, University of Pennsylvania, Philadelphia, PA 19104, USA c John D. Cooper Archaeological and Paleontological Center, Department of Geological Sciences, California State University, Fullerton, CA 92834, USA d Department of Biological Sciences, Louisiana State University, Baton Rouge, LA 70803, USA e Department of Environmental Health Science, University of Georgia, Athens, GA 30602, USA f Museum of Vertebrate Zoology, University of California, Berkeley, CA 94720, USA g Mathematical and Computational Biology Department, Harvey Mudd College, 301 Platt Boulevard, Claremont, CA 9171, USA article info abstract Article history: Molecular analyses of turtle relationships have overturned prevailing morphological hypotheses and Received 11 July 2014 prompted the development of a new taxonomy. Here we provide the first genome-scale analysis of turtle Revised 16 October 2014 phylogeny. We sequenced 2381 ultraconserved element (UCE) loci representing a total of 1,718,154 bp of Accepted 28 October 2014 aligned sequence. Our sampling includes 32 turtle taxa representing all 14 recognized turtle families and Available online 4 November 2014 an additional six outgroups. Maximum likelihood, Bayesian, and species tree methods produce a single resolved phylogeny. -

A New European Albian Turtle That Extends the Known Stratigraphic Range of the Pleurosternidae (Paracryptodira)
Cretaceous Research 55 (2015) 74e83 Contents lists available at ScienceDirect Cretaceous Research journal homepage: www.elsevier.com/locate/CretRes A new European Albian turtle that extends the known stratigraphic range of the Pleurosternidae (Paracryptodira) * A. Perez-García a, b, , E. Espílez c, L. Mampel c, L. Alcala c a Centro de Geologia, Faculdade de Ci^encias da Universidade de Lisboa (FCUL), Edificio C6, Campo Grande, 1749-016 Lisbon, Portugal b Grupo de Biología Evolutiva, Facultad de Ciencias, UNED, Paseo Senda del Rey, 9, 28040 Madrid, Spain c Fundacion Conjunto Paleontologico de Teruel-Dinopolis (Museo Aragones de Paleontología), Avda. Sagunto s/n, E-44002 Teruel, Spain article info abstract Article history: Postcranial material corresponding to three specimens of freshwater turtles, from the lower Albian Received 23 December 2014 (upper Lower Cretaceous) of Arino~ (Teruel Province, Spain), is analysed in this paper. This study allows us Accepted in revised form 18 February 2015 to identify the presence of Pleurosternidae (Paracryptodira) outside its known stratigraphic range, from Available online Kimmeridgian to Barremian, and extends its distribution to the Albian. The species from Arino~ represents a new taxon, Toremys cassiopeia gen. et sp. nov., which is the only pleurosternid described so far in post- Keywords: Berriasian levels. Toremys cassiopeia is closely related to other taxa from Europe, to which the Cretaceous Pleurosternidae pleurosternids are restricted. Knowledge about the European freshwater turtle faunas distributed be- Toremys cassiopeia, gen. et sp. nov. fi Lower Cretaceous tween the Barremian and the uppermost Cretaceous is very limited. The new nding provides relevant Albian data on these poorly understood faunas. -

The Common Snapping Turtle, Chelydra Serpentina
The Common Snapping Turtle, Chelydra serpentina Rylen Nakama FISH 423: Olden 12/5/14 Figure 1. The Common Snapping Turtle, one of the most widespread reptiles in North America. Photo taken in Quebec, Canada. Image from https://www.flickr.com/photos/yorthopia/7626614760/. Classification Order: Testudines Family: Chelydridae Genus: Chelydra Species: serpentina (Linnaeus, 1758) Previous research on Chelydra serpentina (Phillips et al., 1996) acknowledged four subspecies, C. s. serpentina (Northern U.S. and Figure 2. Side profile of Chelydra serpentina. Note Canada), C. s. osceola (Southeastern U.S.), C. s. the serrated posterior end of the carapace and the rossignonii (Central America), and C. s. tail’s raised central ridge. Photo from http://pelotes.jea.com/AnimalFact/Reptile/snapturt.ht acutirostris (South America). Recent IUCN m. reclassification of chelonians based on genetic analyses (Rhodin et al., 2010) elevated C. s. rossignonii and C. s. acutirostris to species level and established C. s. osceola as a synonym for C. s. serpentina, thus eliminating subspecies within C. serpentina. Antiquated distinctions between the two formerly recognized North American subspecies were based on negligible morphometric variations between the two populations. Interbreeding in the overlapping range of the two populations was well documented, further discrediting the validity of the subspecies distinction (Feuer, 1971; Aresco and Gunzburger, 2007). Therefore, any emphasis of subspecies differentiation in the ensuing literature should be disregarded. Figure 3. Front-view of a captured Chelydra Continued usage of invalid subspecies names is serpentina. Different skin textures and the distinctive pink mouth are visible from this angle. Photo from still prevalent in the exotic pet trade for C. -

Dermatemys Mawii (The Hicatee, Tortuga Blanca, Or Central American River Turtle): a Working Bibliography
See discussions, stats, and author profiles for this publication at: https://www.researchgate.net/publication/325206006 Dermatemys mawii (The Hicatee, Tortuga Blanca, or Central American River Turtle): A Working Bibliography Article · May 2018 CITATIONS READS 0 521 6 authors, including: Venetia Briggs Sergio C. Gonzalez University of Florida University of Florida 16 PUBLICATIONS 133 CITATIONS 9 PUBLICATIONS 39 CITATIONS SEE PROFILE SEE PROFILE Thomas Rainwater Clemson University 143 PUBLICATIONS 1,827 CITATIONS SEE PROFILE Some of the authors of this publication are also working on these related projects: Crocodiles in the Everglades View project Dissertation: The role of habitat expansion on amphibian community structure View project All content following this page was uploaded by Sergio C. Gonzalez on 17 May 2018. The user has requested enhancement of the downloaded file. 2018 Endangered and ThreatenedCaribbean Species Naturalist of the Caribbean Region Special Issue No. 2 V. Briggs-Gonzalez, S.C. Gonzalez, D. Smith, K. Allen, T.R. Rainwater, and F.J. Mazzotti 2018 CARIBBEAN NATURALIST Special Issue No. 2:1–22 Dermatemys mawii (The Hicatee, Tortuga Blanca, or Central American River Turtle): A Working Bibliography Venetia Briggs-Gonzalez1,*, Sergio C. Gonzalez1, Dustin Smith2, Kyle Allen1, Thomas R. Rainwater3, and Frank J. Mazzotti1 Abstract - Dermatemys mawii (Central American River Turtle), locally known in Belize as the “Hicatee” and in Guatemala and Mexico as Tortuga Blanca, is a large, highly aquatic freshwater turtle that has been extirpated from much of its historical range of southern Mex- ico, northern Guatemala, and lowland Belize. Throughout its restricted range, Dermatemys has been intensely harvested for its meat and eggs and sold in local markets. -

71St Annual Meeting Society of Vertebrate Paleontology Paris Las Vegas Las Vegas, Nevada, USA November 2 – 5, 2011 SESSION CONCURRENT SESSION CONCURRENT
ISSN 1937-2809 online Journal of Supplement to the November 2011 Vertebrate Paleontology Vertebrate Society of Vertebrate Paleontology Society of Vertebrate 71st Annual Meeting Paleontology Society of Vertebrate Las Vegas Paris Nevada, USA Las Vegas, November 2 – 5, 2011 Program and Abstracts Society of Vertebrate Paleontology 71st Annual Meeting Program and Abstracts COMMITTEE MEETING ROOM POSTER SESSION/ CONCURRENT CONCURRENT SESSION EXHIBITS SESSION COMMITTEE MEETING ROOMS AUCTION EVENT REGISTRATION, CONCURRENT MERCHANDISE SESSION LOUNGE, EDUCATION & OUTREACH SPEAKER READY COMMITTEE MEETING POSTER SESSION ROOM ROOM SOCIETY OF VERTEBRATE PALEONTOLOGY ABSTRACTS OF PAPERS SEVENTY-FIRST ANNUAL MEETING PARIS LAS VEGAS HOTEL LAS VEGAS, NV, USA NOVEMBER 2–5, 2011 HOST COMMITTEE Stephen Rowland, Co-Chair; Aubrey Bonde, Co-Chair; Joshua Bonde; David Elliott; Lee Hall; Jerry Harris; Andrew Milner; Eric Roberts EXECUTIVE COMMITTEE Philip Currie, President; Blaire Van Valkenburgh, Past President; Catherine Forster, Vice President; Christopher Bell, Secretary; Ted Vlamis, Treasurer; Julia Clarke, Member at Large; Kristina Curry Rogers, Member at Large; Lars Werdelin, Member at Large SYMPOSIUM CONVENORS Roger B.J. Benson, Richard J. Butler, Nadia B. Fröbisch, Hans C.E. Larsson, Mark A. Loewen, Philip D. Mannion, Jim I. Mead, Eric M. Roberts, Scott D. Sampson, Eric D. Scott, Kathleen Springer PROGRAM COMMITTEE Jonathan Bloch, Co-Chair; Anjali Goswami, Co-Chair; Jason Anderson; Paul Barrett; Brian Beatty; Kerin Claeson; Kristina Curry Rogers; Ted Daeschler; David Evans; David Fox; Nadia B. Fröbisch; Christian Kammerer; Johannes Müller; Emily Rayfield; William Sanders; Bruce Shockey; Mary Silcox; Michelle Stocker; Rebecca Terry November 2011—PROGRAM AND ABSTRACTS 1 Members and Friends of the Society of Vertebrate Paleontology, The Host Committee cordially welcomes you to the 71st Annual Meeting of the Society of Vertebrate Paleontology in Las Vegas. -

Cantor's Giant Softshell Turtle, Pelochelys Cantorii
M Cantor’s Giant Softshell turtle, Pelochelys cantorii Compiler: Ayushi Jain Suggested citation: Jain, A., Das, A., V. Deepak., Cavada-Blanco, F. 2021. A Survival Blueprint for the Cantor’s Giant Softshell Turtle Pelochelys cantorii in India. EDGE of Existence programme, Zoological Society of London, UK 1. STATUS REVIEW 1.1 Taxonomy: Class : Reptilia Order : Testudines Family : Trionychidae Genus : Pelochelys Species : Pelocheys cantorii (Gray, 1864) Common Name : Cantor’s Giant softshell turtle/ Asian Giant softshell turtle/ Local name : Bheemanama, Paala poovan (Malayalam) Synonyms: Pelochelys clivepalmeri (Hoser, 2014), P. cumingii (Gray, 1864), P. poljakowii (Strauch, 1890), P. telstraorum (Hoser, 2014), P. cantoris (Boulenger, 1889) Pelochelys cantorii (Gray, 1864) is one of the three species in the genus Pelochelys. The other two species are P. bibroni and P. signifera known only from Papua New Guinea and Indonesia (Papua), respectively. P. cantorii has a large distribution across south and south-east Asia (Das, 2008). It is among the largest freshwater turtles in the world with adults reaching a carapace length of around 100 cm (Das, 2008). Sexual dimorphism is present with males having longer and thicker tales than females; something common for other softshell turtles. Females are also larger in size than males (Das, 2008). According to the last IUCN Red List of threatened species assessment for the species, Pelochelys cantorii might hide a complex of several different species (ATTWG, 2000) A B Figure 1. An adult Pelochelys cantorii on the banks of Chandragiri river caught as by-catch in a fishing line (A), and a close-up head shot showing the keratinized sheath or “teeth” of the species (B). -

As an Indeterminate Plesiochelyid Turtle
Reinterpretation of the Spanish Late Jurassic “Hispaniachelys prebetica” as an indeterminate plesiochelyid turtle Adán Pérez-García Acta Palaeontologica Polonica 59 (4), 2014: 879-885 doi: http://dx.doi.org/10.4202/app.2012.0115 A partial postcranial skeleton (carapace, plastron, and other poorly preserved elements) of a turtle, from the late Oxfordian of the Betic Range of Spain, has recently been assigned to a new taxon, Hispaniachelys prebetica. This is one of the few European turtle taxa reported from pre-Kimmeridgian levels, and the oldest turtle so far known from southern Europe. The character combination identified in that taxon (including the presence of cleithra, and single cervical scale) did not allow its assignment to Plesiochelyidae, a group of turtles very abundant and diverse in the Late Jurassic of Europe. The revision of the single specimen assigned to this taxon led to the reinterpretation of some of its elements, being reassigned to Plesiochelyidae. This study confirms the presence of Plesiochelyidae in the Oxfordian. However, because the Spanish taxon does not present a unique combination of characters, it is proposed as a nomen dubium. Key words: Testudines, Plesiochelyidae, “Hispaniachelys prebetica”, Oxfordian, Jurassic, Spain. Adán Pérez-García [[email protected]], Centro de Geologia, Faculdade de Ciências da Universidade de Lisboa (FCUL), Edificio C6, Campo Grande, 1749-016 Lisbon, Portugal; Grupo de Biología Evolutiva, Facultad de Ciencias, UNED, C/ Senda del Rey, 9, 28040 Madrid, Spain. This is an open-access article distributed under the terms of the Creative Commons Attribution License (for details please see creativecommons.org), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. -

Nilssonia Leithii (Gray 1872) – Leith's Softshell Turtle
Conservation Biology of Freshwater Turtles and Tortoises: A Compilation Project ofTrionychidae the IUCN/SSC Tortoise— Nilssonia and Freshwater leithii Turtle Specialist Group 075.1 A.G.J. Rhodin, P.C.H. Pritchard, P.P. van Dijk, R.A. Saumure, K.A. Buhlmann, J.B. Iverson, and R.A. Mittermeier, Eds. Chelonian Research Monographs (ISSN 1088-7105) No. 5, doi:10.3854/crm.5.075.leithii.v1.2014 © 2014 by Chelonian Research Foundation • Published 17 February 2014 Nilssonia leithii (Gray 1872) – Leith’s Softshell Turtle INDRANE I L DAS 1, SHASHWAT SI RS I 2, KARTH ik EYAN VASUDE V AN 3, AND B.H.C.K. MURTHY 4 1Institute of Biodiversity and Environmental Conservation, Universiti Malaysia Sarawak, 94300 Kota Samarahan, Sarawak, Malaysia [[email protected]]; 2Turtle Survival Alliance-India, D-1/316 Sector F, Janakipuram, Lucknow 226 021, India [[email protected]]; 3Laboratory for Conservation of Endangered Species, Centre for Cellular and Molecular Biology, Pillar 162, PVNR Expressway, Hyderguda, Hyderabad 500 048, India [[email protected]]; 4Zoological Survey of India, J.L. Nehru Road, Kolkata 700 016, India [[email protected]] SU mm ARY . – Leith’s Softshell Turtle, Nilssonia leithii (Family Trionychidae), is a large turtle, known to attain at least 720 mm in carapace length (bony disk plus fibrocartilage flap), and possibly as much as 1000 mm. The species inhabits the rivers and reservoirs of southern peninsular India, replacing the more familiar Indian Softshell Turtle, N. gangetica, of northern India. The turtle is apparently rare within its range, even within protected areas, which is suspected to be due to a past history of exploitation.