NKG2D Natural Killer Cell Receptor—A Short Description and Potential Clinical Applications
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Enhancing a Natural Killer: Modification of NK Cells for Cancer
cells Review Enhancing a Natural Killer: Modification of NK Cells for Cancer Immunotherapy Rasa Islam 1,2, Aleta Pupovac 1, Vera Evtimov 1, Nicholas Boyd 1, Runzhe Shu 1, Richard Boyd 1 and Alan Trounson 1,2,* 1 Cartherics Pty Ltd., Clayton 3168, Australia; [email protected] (R.I.); [email protected] (A.P.); [email protected] (V.E.); [email protected] (N.B.); [email protected] (R.S.); [email protected] (R.B.) 2 Department of Obstetrics and Gynaecology, Monash University, Clayton 3168, Australia * Correspondence: [email protected] Abstract: Natural killer (NK) cells are potent innate immune system effector lymphocytes armed with multiple mechanisms for killing cancer cells. Given the dynamic roles of NK cells in tumor surveillance, they are fast becoming a next-generation tool for adoptive immunotherapy. Many strategies are being employed to increase their number and improve their ability to overcome cancer resistance and the immunosuppressive tumor microenvironment. These include the use of cytokines and synthetic compounds to bolster propagation and killing capacity, targeting immune-function checkpoints, addition of chimeric antigen receptors (CARs) to provide cancer specificity and genetic ablation of inhibitory molecules. The next generation of NK cell products will ideally be readily available as an “off-the-shelf” product and stem cell derived to enable potentially unlimited supply. However, several considerations regarding NK cell source, genetic modification and scale up first Citation: Islam, R.; Pupovac, A.; need addressing. Understanding NK cell biology and interaction within specific tumor contexts Evtimov, V.; Boyd, N.; Shu, R.; Boyd, will help identify necessary NK cell modifications and relevant choice of NK cell source. -

HLA Mismatching Favoring Host-Versus-Graft NK Cell Activity Via KIR3DL1 Is Associated With
HLA mismatching favoring host-versus-graft NK cell activity via KIR3DL1 is associated with improved outcomes following lung transplantation John R. Greenland1,2,6, Haibo Sun3, Daniel Calabrese2, Tiffany Chong2, Jonathan P. Singer2, Jasleen Kukreja4, Steven R. Hays2, Jeffrey A. Golden2,4, George H. Caughey1,2,5, Jeffrey M. Venstrom2, Raja Rajalinginam3 1 Medical Service, Veterans Affairs Medical Center, San Francisco CA, 94121 2 Department of Medicine, University of California, San Francisco CA, 94143 3 Immunogenetics and Transplantation Laboratory, Department of Surgery, University of California, San Francisco CA, 94143 4 Department of Surgery, University of California, San Francisco CA, 94143 5 Cardiovascular Research Institute, University of California, San Francisco CA, 94143 6 Corresponding author: [email protected] Running Title: HVG NK cell activity in lung transplantation Abbreviations: antigen presenting cell (APC), bronchiolitis obliterans syndrome (BOS), bronchoalveolar lavage (BAL), chronic lung allograft dysfunction (CLAD), confidence interval (CI), forced expiratory volume in 1 second (FEV1), forced vital capacity (FVC), hazard ratio (HR), host-versus-graft (HVG), Killer cell immunoglobulin-like receptor (KIR), Major Histocompatibility Complex (MHC), Natural Killer (NK), restrictive allograft syndrome (RAS), United Network for Organ Sharing (UNOS), University of California, San Francisco (UCSF) Abstract Chronic lung allograft dysfunction (CLAD) is linked to rejection and limits survival following lung transplantation. HLA-Bw4 recipients of HLA-Bw6 grafts have enhanced host-versus-graft (HVG) NK cell activity mediated by KIR3DL1 ligand. Because natural killer (NK) cells may promote tolerance by depleting antigen-presenting cells, we hypothesized improved outcomes for HLA-Bw4 recipients of HLA- Bw6 grafts. We evaluated differences in acute cellular rejection (ACR) and CLAD-free survival across 252 KIR3DL1+ recipients from UCSF. -

Human and Mouse CD Marker Handbook Human and Mouse CD Marker Key Markers - Human Key Markers - Mouse
Welcome to More Choice CD Marker Handbook For more information, please visit: Human bdbiosciences.com/eu/go/humancdmarkers Mouse bdbiosciences.com/eu/go/mousecdmarkers Human and Mouse CD Marker Handbook Human and Mouse CD Marker Key Markers - Human Key Markers - Mouse CD3 CD3 CD (cluster of differentiation) molecules are cell surface markers T Cell CD4 CD4 useful for the identification and characterization of leukocytes. The CD CD8 CD8 nomenclature was developed and is maintained through the HLDA (Human Leukocyte Differentiation Antigens) workshop started in 1982. CD45R/B220 CD19 CD19 The goal is to provide standardization of monoclonal antibodies to B Cell CD20 CD22 (B cell activation marker) human antigens across laboratories. To characterize or “workshop” the antibodies, multiple laboratories carry out blind analyses of antibodies. These results independently validate antibody specificity. CD11c CD11c Dendritic Cell CD123 CD123 While the CD nomenclature has been developed for use with human antigens, it is applied to corresponding mouse antigens as well as antigens from other species. However, the mouse and other species NK Cell CD56 CD335 (NKp46) antibodies are not tested by HLDA. Human CD markers were reviewed by the HLDA. New CD markers Stem Cell/ CD34 CD34 were established at the HLDA9 meeting held in Barcelona in 2010. For Precursor hematopoetic stem cell only hematopoetic stem cell only additional information and CD markers please visit www.hcdm.org. Macrophage/ CD14 CD11b/ Mac-1 Monocyte CD33 Ly-71 (F4/80) CD66b Granulocyte CD66b Gr-1/Ly6G Ly6C CD41 CD41 CD61 (Integrin b3) CD61 Platelet CD9 CD62 CD62P (activated platelets) CD235a CD235a Erythrocyte Ter-119 CD146 MECA-32 CD106 CD146 Endothelial Cell CD31 CD62E (activated endothelial cells) Epithelial Cell CD236 CD326 (EPCAM1) For Research Use Only. -

And NK-Cell Neoplasms
J Hematopathol (2009) 2:65–73 DOI 10.1007/s12308-009-0034-z COMMENT Commentary on the 2008 WHO classification of mature T- and NK-cell neoplasms Megan S. Lim & Laurence de Leval & Leticia Quintanilla-Martinez Received: 29 April 2009 /Accepted: 1 May 2009 /Published online: 27 June 2009 # Springer-Verlag 2009 Abstract In 2008, the World Health Organization (WHO) Introduction published a revised and updated edition of the classification of tumors of the hematopoietic and lymphoid tissues. The The 2008 World Health Organization (WHO) classification aims of the fourth edition of the WHO classification was to of mature thymus (T)- and natural killer (NK)-cell neo- incorporate new scientific and clinical information in order plasms provide clarification regarding diagnostic criteria, to refine diagnostic criteria for previously described neo- etiologic insights, and prognostic information for several plasms and to introduce newly recognized disease entities. new/provisional entities [1]. The recognition that T-cell The recognition that T-cell lymphomas are related to the lymphomas are related to the innate and adaptive immune innate and adaptive immune system, as well as enhanced system, as well as enhanced understanding of other T-cell understanding of other T-cell subsets, such as the regula- subsets, such as the regulatory T-cell and follicular helper tory T-cell and follicular helper T-cells, has contributed to T-cells (TFH), has contributed to our understanding of the our understanding of the morphologic, histologic, and morphologic, histologic, and immunophenotypic features of immunophenotypic features of T- and NK-cell neoplasms. T- and NK-cell neoplasms. New insights regarding immu- The purpose of this review is to highlight major changes in nophenotypic features of large granular lymphocytes and the classification of T- and NK-cell neoplasms and to NK-cells have provided better flow cytometric tools for the explain the rationale for these changes. -

Downloaded the Normalized Gene Expression Profile from the GEO (Package Geoquery (5))
1 SUPPLEMENTARY MATERIALS 2 Supplementary Methods 3 Study design 4 The workflow to identify and validate the TME risk score and TME subtypes in gastric cancer is 5 depicted in Fig. S1. We first estimated the absolute abundance levels of the major stromal and 6 immune cell types in the TME using bulk gene expression data, and assessed the prognostic 7 effect of these cells in a discovery cohort. Next, we constructed a TME risk score and validated it 8 in two independent gene expression validation cohorts and three immunohistochemistry 9 validation cohorts. Finally, we stratified patients into four TME subtypes and examined their 10 genomic and molecular features and relation to established molecular subtypes. 11 Gene expression data 12 To explore the prognostic landscape of the TME, we used four gene expression profile (GEP) 13 datasets of resected gastric cancer patients with publicly available clinical information, namely, 14 ACRG (GSE62254) (1), GSE15459 (2), GSE84437 (3), and TCGA stomach adenocarcinoma 15 (STAD). Specifically, the raw microarray data in the ACRG cohort and GSE15459 were retrieved 16 from the Gene Expression Omnibus (GEO), and normalized by the RMA algorithm (package affy) 17 using custom chip definition files (Brainarray version 23 (4)) that convert Affymetrix probesets to 18 Entrez gene IDs. For GSE84437 dataset, which was measured by the Illumina platform, we 19 downloaded the normalized gene expression profile from the GEO (package GEOquery (5)). The 20 Illumina probes were also mapped to Entrez genes. For multiple probes mapping to the same 21 Entrez gene, we selected the one with the maximum mean expression level as the surrogate for 22 the Entrez gene using the function of collapseRows (6) (package WGCNA). -
![Anti-NCR1 Antibody [29A1.4] (FITC) (ARG23568)](https://docslib.b-cdn.net/cover/3482/anti-ncr1-antibody-29a1-4-fitc-arg23568-113482.webp)
Anti-NCR1 Antibody [29A1.4] (FITC) (ARG23568)
Product datasheet [email protected] ARG23568 Package: 50 μg anti-NCR1 antibody [29A1.4] (FITC) Store at: 4°C Summary Product Description FITC-conjugated Rat Monoclonal antibody [29A1.4] recognizes NCR1. Rat anti Mouse CD335 antibody, clone 29A1.4 recognizes natural killer cell p46-related protein (NKp46), otherwise known as CD335, which is uniquely expressed by resting and activated natural killer (NK) cells, but no expression of CD335 has been detected on B cells, T cells, monocytes or granulocytes. It is a major NK cell lysis receptor for autologous pathogen-infected and tumor target cells during natural cytotoxicity responses. Ligands of CD335 include viral hemagglutinins (HAs) and heparan sulfate proteoglycans (HSPGs) on the surface of tumor cells. Staining with Rat anti mouse CD335 (29A1.4) is not strain specific and the antibody has been used to stain C57BL/6, SJL, CBA/CA and BALB/c strains. Clone 29A1.4 also activates NK cells in vitro. It does not deplete NK cells in vivo. Tested Reactivity Ms Tested Application FACS Host Rat Clonality Monoclonal Clone 29A1.4 Isotype IgG2a Target Name NCR1 Antigen Species Mouse Immunogen NKP46-IgG1 Fc fusion protein. Conjugation FITC Alternate Names CD antigen CD335; Natural killer cell p46-related protein; Lymphocyte antigen 94 homolog; Natural cytotoxicity triggering receptor 1; hNKp46; NKP46; NKp46; NK cell-activating receptor; LY94; CD335; NK- p46 Application Instructions Application table Application Dilution FACS 1:2 - 1:5 Application Note FACS: Use 10 µl of the suggested working dilution to label 10^6 cells in 100 µl. * The dilutions indicate recommended starting dilutions and the optimal dilutions or concentrations should be determined by the scientist. -

NKG2D Signaling Within the Pancreatic Islets Reduces NOD Diabetes and Increases Protective Central Memory CD81 T-Cell Numbers
Diabetes Volume 69, August 2020 1749 NKG2D Signaling Within the Pancreatic Islets Reduces NOD Diabetes and Increases Protective Central Memory CD81 T-Cell Numbers Andrew P. Trembath,1 Kelsey L. Krausz,1 Neekun Sharma,1 Ivan C. Gerling,2 Clayton E. Mathews,3 and Mary A. Markiewicz1 Diabetes 2020;69:1749–1762 | https://doi.org/10.2337/db19-0979 NKG2D is implicated in autoimmune diabetes. How- The immune receptor NKG2D, expressed by natural killer ever, the role of this receptor in diabetes pathogenesis (NK) cells and subsets of T cells (1–5), is implicated in type IMMUNOLOGY AND TRANSPLANTATION is unclear owing to conflicting results with studies in- 1 diabetes development. However, its role in disease pro- volving global inhibition of NKG2D signaling. We found gression remains unclear, with conflicting reports describ- that NKG2D and its ligands are present in human pan- ing pathogenic (6,7), nonpathogenic (8), and protective (9) creata, with expression of NKG2D and its ligands in- effects of NKG2D signaling. creased in the islets of patients with type 1 diabetes. NKG2D recognizes multiple NKG2D ligands in both To directly assess the role of NKG2D in the pancreas, we humans and mice. These are endogenous ligands, which generated NOD mice that express an NKG2D ligand in are all distantly related to MHC class I in sequence, and are b-islet cells. Diabetes was reduced in these mice. The generally believed to be functionally redundant (10,11). reduction corresponded with a decrease in the effector 1 NKG2D ligands are considered stress ligands, with their to central memory CD8 T-cell ratio. -

Expression Tissue
The Journal of Immunology Killer Cell Ig-Like Receptor and Leukocyte Ig-Like Receptor Transgenic Mice Exhibit Tissue- and Cell-Specific Transgene Expression1 Danny Belkin,* Michaela Torkar,* Chiwen Chang,* Roland Barten,* Mauro Tolaini,† Anja Haude,* Rachel Allen,* Michael J. Wilson,* Dimitris Kioussis,† and John Trowsdale2* To generate an experimental model for exploring the function, expression pattern, and developmental regulation of human Ig-like activating and inhibitory receptors, we have generated transgenic mice using two human genomic clones: 52N12 (a 150-Kb clone encompassing the leukocyte Ig-like receptor (LILR)B1 (ILT2), LILRB4 (ILT3), and LILRA1 (LIR6) genes) and 1060P11 (a 160-Kb clone that contains ten killer cell Ig-like receptor (KIR) genes). Both the KIR and LILR families are encoded within the leukocyte receptor complex, and are involved in immune modulation. We have also produced a novel mAb to LILRA1 to facilitate expression studies. The LILR transgenes were expressed in a similar, but not identical, pattern to that observed in humans: LILRB1 was expressed in B cells, most NK cells, and a small number of T cells; LILRB4 was expressed in a B cell subset; and LILRA1 was found on a ring of cells surrounding B cell areas on spleen sections, consistent with other data showing monocyte/macrophage expression. KIR transgenic mice showed KIR2DL2 expression on a subset of NK cells and T cells, similar to the pattern seen in humans, and expression of KIR2DL4, KIR3DS1, and KIR2DL5 by splenic NK cells. These observations indicate that linked regulatory elements within the genomic clones are sufficient to allow appropriate expression of KIRs in mice, and illustrate that the presence of the natural ligands for these receptors, in the form of human MHC class I proteins, is not necessary for the expression of the KIRs observed in these mice. -

Receptor Nkp46 Cells by the NK Β Murine Pancreatic Recognition And
Recognition and Killing of Human and Murine Pancreatic β Cells by the NK Receptor NKp46 This information is current as Chamutal Gur, Jonatan Enk, Sameer A. Kassem, Yaron of September 27, 2021. Suissa, Judith Magenheim, Miri Stolovich-Rain, Tomer Nir, Hagit Achdout, Benjamin Glaser, James Shapiro, Yaakov Naparstek, Angel Porgador, Yuval Dor and Ofer Mandelboim J Immunol 2011; 187:3096-3103; Prepublished online 17 Downloaded from August 2011; doi: 10.4049/jimmunol.1101269 http://www.jimmunol.org/content/187/6/3096 http://www.jimmunol.org/ Supplementary http://www.jimmunol.org/content/suppl/2011/08/18/jimmunol.110126 Material 9.DC1 References This article cites 29 articles, 9 of which you can access for free at: http://www.jimmunol.org/content/187/6/3096.full#ref-list-1 Why The JI? Submit online. by guest on September 27, 2021 • Rapid Reviews! 30 days* from submission to initial decision • No Triage! Every submission reviewed by practicing scientists • Fast Publication! 4 weeks from acceptance to publication *average Subscription Information about subscribing to The Journal of Immunology is online at: http://jimmunol.org/subscription Permissions Submit copyright permission requests at: http://www.aai.org/About/Publications/JI/copyright.html Email Alerts Receive free email-alerts when new articles cite this article. Sign up at: http://jimmunol.org/alerts The Journal of Immunology is published twice each month by The American Association of Immunologists, Inc., 1451 Rockville Pike, Suite 650, Rockville, MD 20852 Copyright © 2011 by The American Association of Immunologists, Inc. All rights reserved. Print ISSN: 0022-1767 Online ISSN: 1550-6606. The Journal of Immunology Recognition and Killing of Human and Murine Pancreatic b Cells by the NK Receptor NKp46 Chamutal Gur,*,†,1 Jonatan Enk,*,1 Sameer A. -
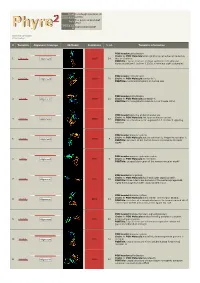
Phyre 2 Results for A1YIY0
Email [email protected] Description A1YIY0 Tue Jul 30 13:19:15 BST Date 2013 Unique Job 1035bc4b501530df ID Detailed template information # Template Alignment Coverage 3D Model Confidence % i.d. Template Information PDB header:cell adhesion Chain: A: PDB Molecule:down syndrome cell adhesion molecule 1 c3dmkA_ Alignment 100.0 14 (dscam) isoform PDBTitle: crystal structure of down syndrome cell adhesion molecule (dscam)2 isoform 1.30.30, n-terminal eight ig domains PDB header:cell adhesion 2 c2om5A_ Alignment 100.0 20 Chain: A: PDB Molecule:contactin 2; PDBTitle: n-terminal fragment of human tax1 PDB header:cell adhesion 3 c3jxaA_ Alignment 100.0 21 Chain: A: PDB Molecule:contactin 4; PDBTitle: immunoglobulin domains 1-4 of mouse cntn4 PDB header:signaling protein/transferase Chain: A: PDB Molecule:tek tyrosine kinase variant; 4 c4k0vA_ 100.0 12 Alignment PDBTitle: structural basis for angiopoietin-1 mediated signaling initiation PDB header:immune system Chain: A: PDB Molecule:natural cytotoxicity triggering receptor 1; 5 c1p6fA_ 100.0 9 Alignment PDBTitle: structure of the human natural cytotoxicity receptor nkp46 PDB header:immune system/receptor 6 c1ollA_ Alignment 99.9 9 Chain: A: PDB Molecule:nk receptor; PDBTitle: extracellular region of the human receptor nkp46 PDB header:viral protein Chain: A: PDB Molecule:hoc head outer capsid protein; 7 c3shsA_ 99.9 18 Alignment PDBTitle: three n-terminal domains of the bacteriophage rb49 highly immunogenic2 outer capsid protein (hoc) PDB header:immune system Chain: E: PDB Molecule:natural -

Single-Cell Profiling Identifies Impaired Adaptive NK Cells Expanded After HCMV Reactivation in Haploidentical HSCT
Single-cell profiling identifies impaired adaptive NK cells expanded after HCMV reactivation in haploidentical HSCT Elisa Zaghi, … , Enrico Lugli, Domenico Mavilio JCI Insight. 2021;6(12):e146973. https://doi.org/10.1172/jci.insight.146973. Research Article Hematology Immunology Graphical abstract Find the latest version: https://jci.me/146973/pdf RESEARCH ARTICLE Single-cell profiling identifies impaired adaptive NK cells expanded after HCMV reactivation in haploidentical HSCT Elisa Zaghi,1 Michela Calvi,1,2 Simone Puccio,3 Gianmarco Spata,1 Sara Terzoli,1 Clelia Peano,4 Alessandra Roberto,3 Federica De Paoli,3 Jasper J.P. van Beek,3 Jacopo Mariotti,5 Chiara De Philippis,5 Barbara Sarina,5 Rossana Mineri,6 Stefania Bramanti,5 Armando Santoro,5 Vu Thuy Khanh Le-Trilling,7 Mirko Trilling,7 Emanuela Marcenaro,8 Luca Castagna,5 Clara Di Vito,1,2 Enrico Lugli,3,9 and Domenico Mavilio1,2 1Unit of Clinical and Experimental Immunology, IRCCS Humanitas Research Hospital, Rozzano, Milan, Italy. 2BIOMETRA, Università degli Studi di Milano, Milan, Italy. 3Laboratory of Translational Immunology, 4Institute of Genetic and Biomedical Research, UoS Milan, National Research Council, and Genomic Unit, 5Bone Marrow Transplant Unit, and 6Molecular Biology Section, Clinical Investigation Laboratory, IRCCS Humanitas Research Hospital, Milan, Italy. 7Institute for Virology, University Hospital Essen, University Duisburg-Essen, Essen, Germany. 8Department of Experimental Medicine, University of Genoa, Genoa, Italy. 9Flow Cytometry Core, IRCCS Humanitas Research Hospital, Milan, Italy. Haploidentical hematopoietic stem cell transplantation (h-HSCT) represents an efficient curative approach for patients affected by hematologic malignancies in which the reduced intensity conditioning induces a state of immunologic tolerance between donor and recipient. -

Receptor Nkp46/NCR1 Tumors in the Absence of the NK-Activating
The Journal of Immunology Enhanced In Vivo Growth of Lymphoma Tumors in the Absence of the NK-Activating Receptor NKp46/NCR11 Gili G. Halfteck, Moran Elboim, Chamutal Gur, Hagit Achdout, Hormas Ghadially, and Ofer Mandelboim2 The in vitro elimination of virus-infected and tumor cells by NK cells is regulated by a balance between signals conveyed via specific inhibitory and activating receptors. Whether NK cells and specifically the NK-activating receptor NKp46 (NCR1 in mice) are directly involved in tumor eradication in vivo is still largely unknown. Since the NKp46/NCR1 tumor ligands have not been identified yet, we use a screening technique to identify functional ligands for NKp46/NCR1 which is based on a cell reporter assay and discover a NCR1 ligand in the PD1.6 lymphoma line. To study whether NKp46/NCR1 is important for the eradication of PD1.6 lymphoma in vivo, we used the Ncr1 knockout Ncr1gfp/gfp mice generated by our group. Strikingly, all Ncr1 knockout mice developed growing PD1.6 tumors, whereas initial tumor growth was observed in the wild-type mice and tumors were completely rejected as time progressed. The growth of other lymphoma cell lines such as B10 and EL4 was equivalent between the Ncr1 knockout and wild-type mice. Finally, we show that PD1.6 lymphoma cells are less killed both in vitro and in vivo in the absence of NKp46/NCR1. Our results therefore reveal a crucial role for NKp46/NCR1 in the in vivo eradication of some lymphoma cells. The Journal of Immunology, 2009, 182: 2221–2230. t was hypothesized that the immune system surveys the body reduced resistance to transplanted tumor cell lines (20–22).