Phase Chemistr\ in Process Models for Cement Clinker and Lime Production
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Environmental, Health, and Safety Guidelines for Cement and Lime Manufacturing
Environmental, Health, and Safety Guidelines CEMENT AND LIME MANUFACTURING WORLD BANK GROUP DRAFT FOR PUBLIC CONSULTATION—AUGUST 2018 Environmental, Health, and Safety Guidelines for Cement and Lime Manufacturing Introduction 1. The Environmental, Health, and Safety (EHS) Guidelines are technical reference documents with general and industry-specific examples of Good International Industry Practice (GIIP).1 When one or more members of the World Bank Group are involved in a project, these EHS Guidelines are applied as required by their respective policies and standards. These industry sector EHS guidelines are designed to be used together with the General EHS Guidelines document, which provides guidance to users on common EHS issues potentially applicable to all industry sectors. For complex projects, use of multiple industry sector guidelines may be necessary. A complete list of industry sector guidelines can be found at www.ifc.org/ehsguidelines. 2. The EHS Guidelines contain the performance levels and measures that are generally considered to be achievable in new facilities by existing technology at reasonable costs. Application of the EHS Guidelines to existing facilities may involve the establishment of site-specific targets, with an appropriate timetable for achieving them. 3. The applicability of the EHS Guidelines should be tailored to the hazards and risks established for each project on the basis of the results of an environmental assessment in which site-specific variables, such as host country context, assimilative capacity of the environment, and other project factors, are taken into account. The applicability of specific technical recommendations should be based on the professional opinion of qualified and experienced persons. -

Manuscript Details
Manuscript Details Manuscript number MATERIALSCHAR_2019_439_R1 Title THE EFFECTS OF MgO, Na2O AND SO3 ON INDUSTRIAL CLINKERING PROCESS: PHASE COMPOSITION, POLYMORPHISM, MICROSTRUCTURE AND HYDRATION, USING A MULTIDISCIPLINARY APPROACH. Article type Research Paper Abstract The present investigation deals with how minor elements (their oxides: MgO, Na2O and SO3) in industrial kiln feeds affect (i) chemical reactions upon clinkering, (ii) resulting phase composition and microstructure of clinker, (iii) hydration process during cement production. Our results show that all these points are remarkably sensitive to the combination and interference effects between the minor chemical species mentioned above. Upon clinkering, all the industrial raw meals here used exhibit the same formation temperature and amount of liquid phase. Minor elements are preferentially hosted by secondary phases, such as periclase. Conversely, the growth rate of the main clinker phases (alite and belite) is significantly affected by the nature and combination of minor oxides. MgO and Na2O give a very fast C3S formation rate at T > 1450 K, whereas Na2O and SO3 boost C2S. After heating, if SO3 occurs in combination with MgO and/or Na2O, it does not inihibit the C3S crystallisation as expected. Rather, it promotes the stabilisation of M1-C3S, thus indirectly influencing the aluminate content, too. MgO increseases the C3S amount and promotes the stabilisation of M3-C3S, when it is in combination with Na2O. Na2O seems to be mainly hosted by calcium aluminate structure, but it does not induce the stabilisation of the orhtorhombic polymorph, as supposed to occur. Such features play a key role in predicting the physical-mechanical performance of a final cement (i.e. -
Cement Data Sheet
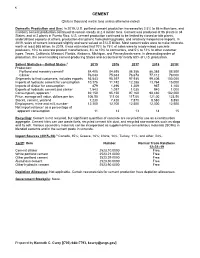
42 CEMENT (Data in thousand metric tons unless otherwise noted) Domestic Production and Use: In 2019, U.S. portland cement production increased by 2.5% to 86 million tons, and masonry cement production continued to remain steady at 2.4 million tons. Cement was produced at 96 plants in 34 States, and at 2 plants in Puerto Rico. U.S. cement production continued to be limited by closed or idle plants, underutilized capacity at others, production disruptions from plant upgrades, and relatively inexpensive imports. In 2019, sales of cement increased slightly and were valued at $12.5 billion. Most cement sales were to make concrete, worth at least $65 billion. In 2019, it was estimated that 70% to 75% of sales were to ready-mixed concrete producers, 10% to concrete product manufactures, 8% to 10% to contractors, and 5% to 12% to other customer types. Texas, California, Missouri, Florida, Alabama, Michigan, and Pennsylvania were, in descending order of production, the seven leading cement-producing States and accounted for nearly 60% of U.S. production. Salient Statistics—United States:1 2015 2016 2017 2018 2019e Production: Portland and masonry cement2 84,405 84,695 86,356 86,368 88,500 Clinker 76,043 75,633 76,678 77,112 78,000 Shipments to final customers, includes exports 93,543 95,397 97,935 99,406 100,000 Imports of hydraulic cement for consumption 10,376 11,742 12,288 13,764 15,000 Imports of clinker for consumption 879 1,496 1,209 967 1,100 Exports of hydraulic cement and clinker 1,543 1,097 1,035 940 1,000 Consumption, apparent3 92,150 95,150 97,160 98,480 102,000 Price, average mill value, dollars per ton 106.50 111.00 117.00 121.00 123.50 Stocks, cement, yearend 7,230 7,420 7,870 8,580 8,850 Employment, mine and mill, numbere 12,300 12,700 12,500 12,300 12,500 Net import reliance4 as a percentage of apparent consumption 11 13 13 14 15 Recycling: Cement is not recycled, but significant quantities of concrete are recycled for use as a construction aggregate. -

96 Quality Control of Clinker Products by SEM and XRF Analysis Ziad Abu
ACXRI '96 Quality Control of Clinker Products By SEM and XRF Analysis Ziad Abu Kaddourah and Khairun Azizi MY9700786 School of Materials and Mineral Resources Eng., Universiti Sains Malaysia 31750 Tronoh, Perak, Malaysia. ABSTRACT The microstructure and chemical properties of industrial Portland cement clinkers have been examined by SEM and XRF methods to establish the nature of the clinkers and how variations in the clinker characteristics can be used to control the clinker quality. The clinker nodules were found to show differences in the chemical composition and microstructure between the inner and outer parts of the clinker nodules. Microstructure studies of industrial Portland cement clinker have shown that the outer part of the nodules are enriched in silicate more than the inner part. There is better crystallization and larger alite crystal 9ize in the outer part than in the inner part. The alite crystal size varied between 16.2-46.12um. The clinker chemical composition was found to affect the residual >45um, where a higher belite content causes an increase in the residual >45um in the cement product and will cause a decrease in the concrete strength of the cement product. The aluminate and ferrite crystals and the microcracks within the alite crystal are clear in some clinker only. The quality of the raw material preparation, burning and cooling stages can be controlled using the microstructure of the clinker product. INTRODUCTION Examination of manufactured industrial clinkers using the Scanning Electron Microscope (SEM) is usually conducted to study problems that can't be defined by the normal quality control procedures. Such a study can be used to give better information and knowledge about clinkers characteristics and how variations in the clinker characteristics are affected by variations in the various stages during the manufacturing process. -

Thermodynamics of Portland Cement Clinkering
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Aberdeen University Research Archive Thermodynamics of Portland Cement Clinkering Theodore Hanein1, Fredrik P. Glasser2, Marcus Bannerman1* 1. School of Engineering, University of Aberdeen, AB24 3UE, United Kingdom 2. Department of Chemistry, University of Aberdeen, AB24 3UE, United Kingdom Abstract The useful properties of cement arise from the assemblage of solid phases present within the cement clinker. The phase proportions produced from a given feedstock are often predicted using well-established stoichiometric relations, such as the Bogue equations. These approaches are based on a single estimation of the stable phases produced under standard processing conditions and so they are limited in their general application. This work presents a thermodynamic database and simple equilibrium model which is capable of predicting cement phase stability across the full range of kiln temperatures, including the effect of atmospheric conditions. This is termed a “reaction path” and benchmarks the kinetics of processes occurring in the kiln. Phase stability is calculated using stoichiometric phase data and Gibbs free energy minimization under the constraints of an elemental balance. Predictions of stable phases and standard phase diagrams are reproduced for the manufacture of ordinary Portland cement and validated against results in the literature. The stability of low-temperature phases, which may be important in kiln operation, is explored. Finally, an outlook on future applications of the database in optimizing cement plant operation and development of new cement formulations is provided. Originality This report details corrections to reference thermodynamic data which are relevant for cement thermodynamic calculations. -

Portland Cement Clinker
Conforms to HazCom 2012/United States Safety Data Sheet Portland Cement Clinker Section 1. Identification GHS product identifier: Portland Cement Clinker Chemical name: Calcium compounds, calcium silicate compounds, and other calcium compounds containing iron and aluminum make up the majority of this product. Other means of identification: Clinker, Cement Clinker Relevant identified uses of the substance or mixture and uses advised against: Raw material for cement manufacturing. Supplier’s details: 300 E. John Carpenter Freeway, Suite 1645 Irving, TX 75062 (972) 653-5500 Emergency telephone number (24 hours): CHEMTREC: (800) 424-9300 Section 2. Hazards Identification Overexposure to portland cement clinker can cause serious, potentially irreversible skin or eye damage in the form of chemical (caustic) burns, including third degree burns. The same serious injury can occur if wet or moist skin has prolonged contact exposure to dry portland cement clinker. OSHA/HCS status: This material is considered hazardous by the OSHA Hazard Communication Standard (29 CFR 1910.1200). Classification of the SKIN CORROSION/IRRITATION – Category 1 substance or mixture: SERIOUS EYE DAMAGE/EYE IRRITATION – Category 1 SKIN SENSITIZATION – Category 1 CARCINOGENICITY/INHALATION – Category 1A SPECIFIC TARGET ORGAN TOXICITY (SINGLE EXPOSURE) [Respiratory tract irritation] – Category 3 GHS label elements Hazard pictograms: Signal word: Danger Hazard statements: Causes severe skin burns and eye damage. May cause an allergic skin reaction. May cause respiratory irritation. May cause cancer. Precautionary statements: Prevention: Obtain special instructions before use. Do not handle until all safety precautions have been read and understood. Avoid breathing dust. Use outdoors in a well ventilated area. Wash any exposed body parts thouroughly after handling. -

CBA® Quality/Strength-Enhancing Additive
PRODUCT DATA SHEET CBA® Quality/strength-enhancing additive Product Description The CBA® series of additives is a new concept in grinding aids - significantly different from any previous GCP product for the cement industry. The difference is that it is a “Grinding Aid Plus”. CBA additives offer all the benefits of traditional grinding aids, such as increased grinding efficiency and cement flowability. In addition, they offer a unique ability to improve cement strengths which might otherwise be deficient due to mechanical, physical or chemical shortcomings. Advantages include either reduced cost of production or increased cement strengths. Physical Properties Product specifications for the most widely used CBA formulations are as follows: Product SG pH CBA 1102 1.06 (±0.01) 7 - 9 CBA 1104 1.07 (±0.01) 8 - 12 CBA 1115 1.10 (±0.01) 6 - 8 Specifications for other CBA formulations not shown above are available through GCP Applied Technologies Field Engineers. • Increased early and long-term compressive strengths for production of better quality cements. Primary Benefit • Reduced cost of cement production through reduced unit grinding The CBA series of additives consists of tailor-made formulations costs and through replacement of clinker with reactive additions to optimise performance and meet specific requirements at each such as pozzolans, blast furnace slag and fly ash or with fillers such plant. as limestone. The use of CBA allows the cement producer to reduce fineness • CBA additives are most effective in enhancing compressive and achieve lower unit power costs without sacrificing strength. strengths of blended cements using up to 40% limestone filler. Compared with conventional grinding aids, CBA offers unit power • When cement particle size is not reduced, the addition of CBA savings of up to 25% with no loss of strength. -

Delayed Ettringite Formation
Ettringite Formation and the Performance of Concrete In the mid-1990’s, several cases of premature deterioration of concrete pavements and precast members gained notoriety because of uncertainty over the cause of their distress. Because of the unexplained and complex nature of several of these cases, considerable debate and controversy have arisen in the research and consulting community. To a great extent, this has led to a misperception that the problems are more prevalent than actual case studies would indicate. However, irrespective of the fact that cases of premature deterioration are limited, it is essential to address those that have occurred and provide practical, technically sound solutions so that users can confidently specify concrete in their structures. Central to the debate has been the effect of a compound known as ettringite. The objectives of this paper are: Fig. 1. Portland cements are manufactured by a process that combines sources of lime (such as limestone), silica and • to define ettringite and its form and presence in concrete, alumina (such as clay), and iron oxide (such as iron ore). Appropriately proportioned mixtures of these raw materials • to respond to questions about the observed problems and the are finely ground and then heated in a rotary kiln at high various deterioration mechanisms that have been proposed, and temperatures, about 1450 °C (2640 °F), to form cement compounds. The product of this process is called clinker • to provide some recommendations on designing for durable (nodules at right in above photo). After cooling, the clinker is concrete. interground with about 5% of one or more of the forms of Because many of the questions raised relate to cement character- calcium sulfate (gypsum shown at left in photo) to form portland cement. -

Cementing Qualities of the Calcium Aluminates
DEPARTMENT OF COMMERCE Technologic Papers of THE Bureau of Standards S. W. STRATTON, Director No. 19 7 CEMENTING QUALITIES OF THE CALCIUM ALUMINATES BY P. H. BATES, Chemist Bureau of Standards SEPTEMBER 27, 1921 PRICE, 10 CENTS Sold only by the Superintendent of Documents, Government Printing Office Washington, D. C. WASHINGTON GOVERNMENT PRINTING OFFICE 1921 CEMENTING QUALITIES OF THE CALCIUM ALBUMI- NATES Bv P. H. Bates ABSTRACT four calcium aluininates (3CaO.Al 5Ca0.3Al , CaO.Al The 2 3 , 2 3 2 3 , 3Ca0.5Al 2 3 ) have been made in a pure condition and their cementing qualities determined. The first two reacted so energetically with water that too rapid set resulted to make them usable commercially. The last two set more slowly and developed very high strengths at early periods. These two alurninates high in alumina were later made in a pure and impure condition in larger quantities in a rotary kiln and concrete was made from the resulting ground clinker. A 1:1.5:5.5 gravel concrete developed in 24 hours as high strength as a similarly proportioned Portland cement concrete would have developed in 28 days. Recent investigations * have shown that lime can combine with alumina only in the following molecular proportions: 3CaO. A1 5Ca0.3Al , CaO.Al 3Ca0.5Al . These investiga- 2 3 , 2 3 2 3 , 2 3 tions also show that the tricalcium aluminate is the only aluminate present in Portland cement of normal composition and normal properties. Later these results were confirmed by work carried on at this Bureau and published as Technologic Paper No. -

OK™ Cement Mill the Most Energy- Efficient Mill for Cement Grinding
OK™ cement mill The most energy- efficient mill for cement grinding WE DISCOVER POTENTIAL Quality and profit-improving features Application advantages Design advantages Proven commercially, the OK™ mill is the premier roller mill for The OK mill uses a hydro-pneumatic system to press its grinding finish grinding of Portland cement, slag and blended cements. The rollers against the material bed on the rotating grinding table. mill consistently uses five to ten percent less power than other The patented grooved roller profile has two grinding zones, an cement vertical roller mills, and in comparison with traditional ball inner and an outer. The inner zone prepares the grinding bed by mill operations, the energy requirements for the OK cement mill is compressing the feed material as it moves under the rollers into the 30-45 percent lower for cement grinding and 40-50 percent lower high-pressure grinding zone. The center groove allows air to for slag. The OK mill can contribute significantly to profitability and escape from the material. Grinding pressure is concentrated under competitiveness. the outer zone of the roller, allowing for most efficient operation. Segmented roller wear parts are made of the hardest possible The design combines the drying, grinding, material conveying and material without risk of cracking and are very well suited for hard separation processes into just one unit, thus simplifying the plant facing. Re-positioning of rollers is possible for evening out wear. layout. These features ensure maximum longevity. The OK mill incorporates unique patented design elements in the Operating advantages roller and table profile that improve operating stability and reliability, The rollers are in a lifted position when the mill is started, ensuring giving a typical availability of 90 to 95 percent of scheduled ope- trouble-free start-up. -

Item 421 Hydraulic Cement Concrete
421 Item 421 Hydraulic Cement Concrete 1. DESCRIPTION Furnish hydraulic cement concrete for concrete pavements, concrete structures, and other concrete construction. 2. MATERIALS Use materials from prequalified sources listed on the Department website. Provide coarse and fine aggregates from sources listed in the Department’s Concrete Rated Source Quality Catalog (CRSQC). Use materials from non-listed sources only when tested and approved by the Engineer before use. Allow 30 calendar days for the Engineer to sample, test, and report results for non-listed sources. Do not combine approved material with unapproved material. 2.1. Cement. Furnish cement conforming to DMS-4600, “Hydraulic Cement.” 2.2. Supplementary Cementing Materials (SCM). Fly Ash. Furnish fly ash, ultra-fine fly ash (UFFA), and modified Class F fly ash (MFFA) conforming to DMS-4610, “Fly Ash.” Slag Cement. Furnish Slag Cement conforming to DMS-4620, “Slag Cement.” Silica Fume. Furnish silica fume conforming to DMS-4630, “Silica Fume.” Metakaolin. Furnish metakaolin conforming to DMS-4635, “Metakaolin.” 2.3. Cementitious Material. Cementitious materials are the cement and supplementary cementing materials used in concrete. 2.4. Chemical Admixtures. Furnish admixtures conforming to DMS-4640, “Chemical Admixtures for Concrete.” 2.5. Water. Furnish mixing and curing water that is free from oils, acids, organic matter, or other deleterious substances. Water from municipal supplies approved by the Texas Department of Health will not require testing. Provide test reports showing compliance with Table 1 before use when using water from other sources. Water that is a blend of concrete wash water and other acceptable water sources, certified by the concrete producer as complying with the requirements of both Table 1 and Table 2, may be used as mix water. -

Morphological Analysis of White Cement Clinker Minerals: Discussion on the Crystallization-Related Defects
Hindawi Publishing Corporation International Journal of Analytical Chemistry Volume 2016, Article ID 1259094, 10 pages http://dx.doi.org/10.1155/2016/1259094 Research Article Morphological Analysis of White Cement Clinker Minerals: Discussion on the Crystallization-Related Defects Mohamed Benmohamed,1,2 Rabah Alouani,2 Amel Jmayai,1 Abdesslem Ben Haj Amara,1 and Hafsia Ben Rhaiem1 1 UR05/13-01, Physique des Materiaux´ Lamellaires et Nanomateriaux´ Hybrides (PMLNMH), Faculte´ des Sciences de Bizerte, 7021 Zarzouna, Tunisia 2Departement´ de Geologie,´ Faculte´ des Sciences de Bizerte, Zarzouna, 7021 Bizerte, Tunisia Correspondence should be addressed to Mohamed Benmohamed; [email protected] Received 23 January 2016; Accepted 28 April 2016 Academic Editor: Valentina Venuti Copyright © 2016 Mohamed Benmohamed et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. The paper deals with a formation of artificial rock (clinker). Temperature plays the capital role in the manufacturing process. So, itis useful to analyze a poor clinker to identify the different phases and defects associated with their crystallization. X-ray fluorescence spectroscopy was used to determine the clinker’s chemical composition. The amounts of the mineralogical phases are measured by quantitative XRD analysis (Rietveld). Scanning electron microscopy (SEM) was used to characterize the main phases of white Portland cement clinker and the defects associated with the formation of clinker mineral elements. The results of a study which focused on the identification of white clinker minerals and defects detected in these noncomplying clinkers such as fluctuation of the amount of the main phases (alite (C3S) and belite (C2S)), excess of the free lime, occurrence of C3S polymorphs, and occurrence of moderately-crystallized structures are presented in this paper.