A Review of the Genus Bombycomovpha C. F E L D E R & R
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Download This
TOWN PRODUCT CONTACT ATTRIBUTES ACCOMM CONTACT DETAILS DETAILS STEINKOPF Kookfontein Tel: 027-7218841 *Cultural Tours *Kookfontein 027-7218841 Rondawels Faks: 027-7218842 *Halfmens / Succulent Rondawels – Situated along the N7, E-mail: tours self catering / on about 60 km from [email protected] *Flower tours (during request Springbok on the way flower season) to Vioolsdrif, Cultural/field Calitz *Hiking / walking Steinkopf has a strong Guide 0736357021 tours Nama culture due to *Immanual Centre the strong Nama (Succulent Nursery) history inherited from *Kinderlê (sacred mass the past. grave of 32 Nama children) 24 hr Petrol Station; *Steinkopf High ATM / FNB; Surgery; School Choir (songs in Ambulance Service; Nama, Xhosa, German Shops/Take Aways; and Afrikaans Night Club; Pub; *Klipfontein (old Liquor Stores; Police watertower and Anglo Station. Boere War graves of British soldiers) PORT NOLLOTH Municipal Alta Kotze *Port Nolloth *Bedrock 027-851 8353 Offices 027-8511111 Museum *Guesthouse A small pioneering *Port Nolloth *Scotia Inn Hotel: 027-85 1 8865 harbour town on the Seafarms *Port Indigo Guest 027-851 8012 icy cold Atlantic *Harbour – experience house: Ocean, Port Nolloth is the rich history of the *Mcdougalls Bay 027-8511110 home to diamond coastal area Caravan Park & divers, miners and *Sizamile – a township Chalets fishers with with a rich culture and a *Muisvlak Motel: 027-85 1 8046 fascinatingly diverse long history of struggle *Country Club Flats: 0835555919 cultures. *Historical Roman Catholic Church – Self contained ATM/Banking (FNB) near the beach, one of holiday facilities and Service the oldest buildings accommodation: Station; Surgery; around *Daan deWaal 0825615256 Ambulance; Police *Willem *R. -

Aquifer Vulnerability of South Africa
17° 18° 19° 20° 21° 22° 23° 24° 25° 26° 27° 28° 29° 30° 31° 32° Z I M B A B W E 22° 22° Musina Pafuri Mopane Tshipise Alldays Pundu Maria Swartwater Buysdorp Makhado Thohoyandou Tom Burke Levubu 23° 23° Bochum Elim Shingwedzi Mogwadi Giyani Rebone Vivo-Dendron Ga-Ramokgopa Morebeng Lephalale Mooketsi Aquifer Vulnerability POLOKWANE Tzaneen Bakenberg Mmotong Letsitele Seshego PHALABORWA of Gravellotte Olifants E Mokopane 24° 24° Sentrum Dorpsrivier U South Africa Mookgophong Zebediela Nyl River Valley Penge Hoedspruit B O T S W A N A Mookgophong Ga-Masemola Satara Q Thabazimbi Roedtan I Dwaalboom Modimolle Jane Furse Steelpoort Supingstadt Ohrigstad B Crcodile River Bela-Bela Bushbuckridge Northam Marble Hall Belfast Tloonane Village M Rapotokwane Mashishing Skukuza Siyabuswa Sabie Hazyview Motswedi Ga Mokgatlha Mabeskraal Fafung 25° A 25° Groblersdal Roossenekal Mokgola Bagatla Crocodile River Lehurutshe Soshanguve Z Nossob Moloto Dullstroom Komatipoort Zeerust Swartruggens NELSPRUIT Brits Cullinan Malalane O Ottoshoop Rustenburg Kroondal_Marikana Middelburg PRETORIA Bronkhorstspruit Machadodorp Mata-Mata Pomfret Mafikeng Koster Centurion M Tosca eMalahleni Barberton Bo-Molopo Tarlton Lichtenburg Carolina Badplaas Krugersdorp Kempton Park Piet Plessis Delmas 26° JOHANNESBURG Hendrina 26° Heuningvlei Setlagole Ventersdorp-Eye Ventersdorp Springs Carletonville Background: Coligny Leandra Heidelberg Secunda Implementation of the Reconstruction and Development Programme Twee Rivieren Stella Sannieshof Bethal Ganyesa Ermelo Potchefstroom Amsterdam (RDP) in South Africa has highlighted the importance of groundwater Delareyville Vereeniging Balfour resources in the country as the role they will play in satisfying the targets Sasolburg Greylingstad Morgenzon Rietfontein Ottosdal Klerksdorp SWAZILAND Van Zylsrus Migdol of the RDP. As a result, exploration, development and protection of Vryburg Parys Deneysville Standerton Askham Vredefort aquifers is receiving unprecedented attention. -

39 3-2-2016 Gautliquor
THE PROVINCE OF DIE PROVINSIE VAN UNITY DIVERSITY GAUTENG IN GAUTENG Provincial Gazette Provinsiale Koerant EXTRAORDINARY • BUITENGEWOON Selling price • Verkoopprys: R2.50 Other countries • Buitelands: R3.25 PRETORIA Vol. 22 3 FEBRUARY 2016 No. 39 3 FEBRUARIE 2016 We oil Irawm he power to pment kiIDc AIDS HElPl1NE 0800 012 322 DEPARTMENT OF HEALTH Prevention is the cure ISSN 1682-4525 N.B. The Government Printing Works will 00039 not be held responsible for the quality of “Hard Copies” or “Electronic Files” submitted for publication purposes 9 771682 452005 2 No. 39 PROVINCIAL GAZETTE, EXTRAORDINARY, 3 FEBRUARY 2016 1 A message from Government Printing Oit)TV6, Works Notice Submissions Rule: Single notice, single email Dear Valued Customer, Over the last six months, GPW has been experiencing problems with many customers that are still not complying with GPW’s rule of single notice, single email (with proof of payment or purchase order). You are advise that effective from 18 January 2016, all notice submissions received that do no comply with this rule will be failed by our system and your notice will not be processed. In the case where a Z95, Z95Prov or TForm3 Adobe form is submitted with content, there should be a separate Adobe form completed for each notice content which must adhere to the single notice, single email rule. A reminder that documents must be attached separately in your email to GPW. (In other words, your email should have an electronic Adobe Form plus proof of payment/purchase order – 2 separate attachments – where notice content is applicable, it should also be a 3rd separate attachment). -

10 Year Report 1
DOCKDA Rural Development Agency: 1994–2004 Celebrating Ten Years of Rural Development DOCKDA 10 year report 1 A Decade of Democracy 2 Globalisation and African Renewal 2 Rural Development in the Context of Globalisation 3 Becoming a Rural Development Agency 6 Organogram 7 Indaba 2002 8 Indaba 2004 8 Monitoring and Evaluation 9 Donor Partners 9 Achievements: 1994–2004 10 Challenges: 1994–2004 11 Namakwa Katolieke Ontwikkeling (Namko) 13 Katolieke Ontwikkeling Oranje Rivier (KOOR) 16 Hopetown Advice and Development Office (HADO) 17 Bisdom van Oudtshoorn Katolieke Ontwikkeling (BOKO) 18 Gariep Development Office (GARDO) 19 Karoo Mobilisasie, Beplanning en Rekonstruksie Organisasie (KAMBRO) 19 Sectoral Grant Making 20 Capacity Building for Organisational Development 27 Early Childhood Development Self-reliance Programme 29 HIV and AIDS Programme 31 2 Ten Years of Rural Development A Decade of Democracy In 1997, DOCKDA, in a publication summarising the work of the organisation in the first three years of The first ten years of the new democracy in South Africa operation, noted that it was hoped that the trickle-down coincided with the celebration of the first ten years approach of GEAR would result in a steady spread of of DOCKDA’s work in the field of rural development. wealth to poor people.1 In reality, though, GEAR has South Africa experienced extensive changes during failed the poor. According to the Human Development this period, some for the better, some not positive at Report 2003, South Africans were poorer in 2003 than all. A central change was the shift, in 1996, from the they were in 1995.2 Reconstruction and Development Programme (RDP) to the Growth, Employment and Redistribution Strategy Globalisation and African Renewal (GEAR). -

F I N a L CS1 31012007.Indd
MADAGASCAR CONSERVATION & DEVELOPMENT VOLUME 1 | ISSUE 1 — DECEMBER 2006 PAGE 34 REPORT ON A FEASIBILITY STUDY Indigenous silk moth farming as a means to support Ranomafana National Park Tsiresy RazafimanantosoaI, Olga R. RavoahangimalalaI, Correspondence: Catherine L. CraigII Catherine L. Craig 221 Lincoln Road Lincoln, MA 01773 Telephone: +31 781 2599184 E-mail: [email protected] ABSTRACT uct may be wild silk. Wild silk can be sustainably harvested in We envisage a world where the rural poor can derive a livelihood remote areas and easily transported to commercial centers. To from protecting forests instead of cutting them down; where determine if wild silk is a potential means of income generation development planners understand that habitat health is the key- for people living in areas of Madagascar where silk has not been stone for human health and survival, and where conservation traditionally produced, we gathered three types of information: biologists understand that long - term solutions to biodiversity 1. The diversity of silk producing larvae in the Eastern Forest loss must be built around social programs which enable local Corridor and specifically in Ranomafana people to thrive. Our vision, however, can only be achieved 2. The physical properties of larval silk and their estimated com- when scientists express the role of biodiversity conservation mercial value in economic terms (Baird and Dearden 2003), and development 3. How to apply our data in order to select sites where wild silk planners understand environmental complexity and its role in production could have a maximum economic and conservation poverty alleviation. Our long - term goal is to develop a gener- effect alized approach to biodiversity conservation that will enable We emphasize that the work reported here is preliminary scientists and development professionals to identify, plan and and that we are working to expand our database for silkworm initiate sustainable, small - scale businesses in ecologically larvae and potential projects sites. -

Normal Template
6 SOCIO-ECONOMIC BASELINE: 6.1 INTRODUCTION The proposed wind farm project is located within the Northern Cape Province, the Namakwa District Municipality (DM) and the Richtersveld Local Municipality (LM). The Namakwa District Municipality is in the western part of the Northern Cape. The province consists of six local municipalities and covers a geographical area of approximately 126, 747 km². It is bordered by the Siyanda and Pixley ka Seme Districts of the Northern Cape Province to the North-East and East, respectively, and by the Western Cape Province to the South (the West Coast, Boland and Central Karoo District Municipalities). The Atlantic Ocean forms the Western boundary, while the Gariep (Orange) River forms the Northern border with Namibia. The Richtersveld LM comprises of the following towns; Port Nolloth, Lekkersing, Kuboes, Sanddrift, Eksteenfontein, Sendelingsdrift and Alexander Bay. The project site is located on communal land that was formerly the Richtersveld “Coloured Reserve” during the Apartheid era. As part of the transformation and land restitution process, the land has since been transferred back to the ‘Richtersveld community’. For the purpose of this study, the geographic areas described in the socio- economic baseline include the Namakwa DM, Richtersveld LM, and the four rural towns that comprise the Richtersveld community (Lekkersing, Kuboes, Sanddrift and Eksteenfontein) given that they are the beneficiaries of the land on which the proposed site will be located. Figure 4.1 shows the directly affected, neighbouring municipalities and the location of the proposed project site. 6.1.1 Local Population Overview – Relevance of Performance Standard 7: Indigenous People The proposed wind farm project will be located on communal land that was given back to the Richtersveld community as part of the transformation process. -

In the Land Claims Court of South Africa
IN THE LAND CLAIMS COURT OF SOUTH AFRICA Heard at CAPE TOWN during 4-28 September 2000 CASE NUMBER: LCC 151/98 and 23-26 October 2000 before Gildenhuys AJ and Wiechers (assessor) Decided on: 22 March 2001 In the case between: THE RICHTERSVELD COMMUNITY First Plaintiff THE KUBOES COMMUNITY Second Plaintiff THE SANDDRIFT COMMUNITY Third Plaintiff THE LEKKERSING COMMUNITY Fourth Plaintiff THE EKSTEENFONTEIN COMMUNITY Fifth Plaintiff THE ADULT MEMBERS OF THE RICHTERSVELD COMMUNITY Sixth Plaintiff and ALEXKOR LIMITED First Defendant THE GOVERNMENT OF THE REPUBLIC OF SOUTH AFRICA Second Defendant JUDGMENT GILDENHUYS AJ: General background [1] This is an action by inhabitants of the territory commonly known as the Richtersveld, for the restitution of rights in land in respect of a portion of the Richtersveld. The inhabitants allege they were dispossessed of these rights as a result of past racially discriminatory laws or practices. Page 2 The land [2] The Richtersveld is situated in the north-western corner of the Northern Cape Province. It is necessary, for purposes of this case, to identify specific parts of the Richtersveld. In the west there is a narrow strip of land running parallel to the Atlantic Ocean and extending from the Garib River (previously known as the Orange River) in the north, down to White Point (which lies just south of Port Nolloth) in the south. The plaintiffs’ claims for restitution relates to this part of the Richtersveld. I shall refer to it as the subject land.1 The subject land has, following upon the discovery of diamonds on it during the first half of the 20th century, been used for the exploitation of diamonds. -
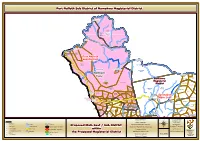
NC Sub Oct2016 N-Portnolloth.Pdf
# # !C # ### # !C^# #!.C# # !C # # # # # # # # # # # ^!C # # # # # # ^ # # ^ # ## # !C ## # # # # # # # # # # # # # # # !C# # !C # # # # # # ## # # #!C# # # # !C# # # # ## ^ ## # # !C # # # # ## # # # #!C # # ^ !C # # # #^ # # # # # # ## ## # # #!C# # # # # # # !C# ## !C# # ## # # # # # !C # !C # # # ###^ # # # # # # # # # # # # !C# ## ## # ## # # # # # # # # ## # # # # #!C ## # # # !C# # # # # # # ## # # # # # # # ##!C ## # # ## !C## # # ## # # ## # ##!C# # # !C# # # #^ # # # # # # # # # # ## # # # # # # # ## # ## # # # # #!C ## #!C #!C# ## # # # # # # # # ^ # # ## # # ## # # !C# ^ ## # # # # ## # # # # # # ## # # # # ## # ## # # # # ## ## # # # # !C# # !C # # #!C # # # #!C ## # # # !C## # # # # ## # # # # # # # ## ## # # ### # # # # # ## # # # # # # ## ###!C # ## ## ## # # ## # # # ### ## # # # ^!C# ### # # # # ^ # # # # # ## ## # # # # # #!C # ### # ## #!C## # #!C # # !C # #!C#### # # ## # # # # !C # # # ## # # # ## # # ## # ## # # ## # ## #!C# # # ## # # # # !C# # ####!C## # # !C # # # #!C ## !C# # !.# # ## # # # # # # ## ## #!C # # # # # ## # # # #### # # ## # # # ## # ## # #^# # # # # ^ ## # !C# ## # # # # # # # !C ## # # # ###!C## ##!C# # # # # ## !C# # !C### # # ^ # !C ##### # # !C# ^##!C# # # !C # #!C## ## ## ## #!C # # ## # # ## # # ## # ## !C # # # ## ## #!C # # # # !C # # ^# ### ## ## ## # # # # !C# !.!C## # !C# ##### ## # # # # ## ## ## ### # !C### # # # # ## #!C## # # ## ### ## # # # # ^ # # ## # # # # # # ## !C# # !C ^ ## # # ^ # # # # ## ^ ## ## # # # # # #!C # !C## # #!C # # # ## ## # # # # # # # ## #!C# # !C # # # !C -

Gauteng Region Our Affiliate Members: Care Homes, Associations and Professionals
Gauteng Region Our affiliate members: care homes, associations and professionals Please note: 1. An important question to ask, at interview stage, is whether the home is equipped to cope regardless of any changes that may occur in the resident's behaviour. Repeatedly moving the person is to be avoided. Some places can't keep people who become disruptive, so it's a good idea to get the reassurance of a commitment to long-term care. 2. Dementia care is specialised care. It's important that the care staff have attended an Alzheimer's SA course in caring for the person with dementia. 3. We urge you to visit the home in person as Alzheimer’s SA cannot be held responsible for the day-to-day running of all its affiliate members. Please see our checklist for choosing a suitable home. Contact Name Area Email/Web Number Gauteng: A Plot 442, Felstead Abbey Cross Lodge (t) 083 228 0933 [email protected] Ave, Northriding Age-in-Action Johannesburg, (t) 011-331 8509 [email protected] Pretoria ADCare SA (t) 012-807 3547 [email protected] Alberton Tuiste vir Alberton 011 907 8307/8 Bejaardes [email protected] B Belenois Forida Hills (t)011 675 4208 [email protected] Retirement Village Blymoedig Clinic Lynne East (t) 012-819 1624 [email protected] Boksburg Society for the Aged Cinda Park (t) 011-915 5416 [email protected] (Cosmos) C Caring at Home Rant-en-dal (t) 082 339 5974 [email protected] Centurion Council Lyttelton (t) 012-664 5754 [email protected] for the Aged Chelsea Lane Rynfield (t) 011-969 6600 [email protected] -
Nc Travelguide 2016 1 7.68 MB
Experience Northern CapeSouth Africa NORTHERN CAPE TOURISM AUTHORITY Tel: +27 (0) 53 832 2657 · Fax +27 (0) 53 831 2937 Email:[email protected] www.experiencenortherncape.com 2016 Edition www.experiencenortherncape.com 1 Experience the Northern Cape Majestically covering more Mining for holiday than 360 000 square kilometres accommodation from the world-renowned Kalahari Desert in the ideas? North to the arid plains of the Karoo in the South, the Northern Cape Province of South Africa offers Explore Kimberley’s visitors an unforgettable holiday experience. self-catering accommodation Characterised by its open spaces, friendly people, options at two of our rich history and unique cultural diversity, finest conservation reserves, Rooipoort and this land of the extreme promises an unparalleled Dronfield. tourism destination of extreme nature, real culture and extreme adventure. Call 053 839 4455 to book. The province is easily accessible and served by the Kimberley and Upington airports with daily flights from Johannesburg and Cape Town. ROOIPOORT DRONFIELD Charter options from Windhoek, Activities Activities Victoria Falls and an internal • Game viewing • Game viewing aerial network make the exploration • Bird watching • Bird watching • Bushmen petroglyphs • Vulture hide of all five regions possible. • National Heritage Site • Swimming pool • Self-drive is allowed Accommodation The province is divided into five Rooipoort has a variety of self- Accommodation regions and boasts a total catering accommodation to offer. • 6 fully-equipped • “The Shooting Box” self-catering chalets of six national parks, including sleeps 12 people sharing • Consists of 3 family units two Transfrontier parks crossing • Box Cottage and 3 open plan units sleeps 4 people sharing into world-famous safari • Luxury Tented Camp destinations such as Namibia accommodation andThis Botswanais the world of asOrange well River as Cellars. -

Process for the Proposed Jukskei Park Pump Station Phase Two Refurbishment in the City of Johannesburg, Gauteng Province
BASIC ASSESSMENT (BA) PROCESS FOR THE PROPOSED JUKSKEI PARK PUMP STATION PHASE TWO REFURBISHMENT IN THE CITY OF JOHANNESBURG, GAUTENG PROVINCE Background Information Document June 2021 AIM OF THIS BACKGROUND INFORMATION DOCUMENT This document aims to provide you, as an interested and/or affected party (I&AP), with: An overview of the proposed development; An overview of the authorisation process and the specialist studies being undertaken to assess the potential impacts, both positive and negative of the proposed project. Details of how you can become involved in the process, receive information, or raise issues which may concern and/or interest you. OVERVIEW OF THE PROPOSED PROJECT Johannesburg Water SOC Ltd which is an entity of the City of Johannesburg (CoJ) has appointed Royal HaskoningDHV (RHDHV) as a service provider to perform an engineering and environmental advisory role for the proposed refurbishment of the Jukskei Pump Station. The Western Klein Jukskei Pumped (WKJP) system consists of four sub-basins which drain to either the Zandspruit- or the Jukskei Park pump stations. From the respective pump stations sewage is pumped to the Western Klein Jukskei outfall (WKJ) which drains to the Northern waste water treatment works (WWTW). Refer to Figure 1 below for details of the WKJP sub-basins. The Jukskei Park Sub-basin gravitates to the Jukskei Park pump station: This sub-basin serves part of the Johannesburg North, Jukskei Park, Noordhang and North Riding comprising mainly residential properties. Joburg Water has identified a need for to refurbish the Jukskei Pump Station due to the dilapidated and poor condition of the infrastructure at the Pump Station. -

Legal Notices Wetlike Kennisgewings
. October Vol. 652 Pretoria, 18 Oktober 2019 No. 42771 LEGAL NOTICES WETLIKE KENNISGEWINGS SALES IN EXECUTION AND OTHER PUBLIC SALES GEREGTELIKE EN ANDER QPENBARE VERKOPE 2 No. 42771 GOVERNMENT GAZETTE, 18 OCTOBER 2019 STAATSKOERANT, 18 OKTOBER 2019 No. 42771 3 CONTENTS / INHOUD LEGAL NOTICES / WETLIKE KENNISGEWINGS SALES IN EXECUTION AND OTHER PUBLIC SALES GEREGTELIKE EN ANDER OPENBARE VERKOPE Sales in execution • Geregtelike verkope ....................................................................................................... 12 Gauteng ...................................................................................................................................... 12 Eastern Cape / Oos-Kaap ................................................................................................................ 55 Free State / Vrystaat ....................................................................................................................... 57 KwaZulu-Natal .............................................................................................................................. 63 Limpopo ...................................................................................................................................... 80 Mpumalanga ................................................................................................................................ 82 North West / Noordwes ................................................................................................................... 86 Northern