Discrimination of Chiral Compounds Using NMR Spectroscopy,” by Thomas J
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Organic Compounds
organic compounds Acta Crystallographica Section E = 1.73 mmÀ1 0.68 Â 0.58 Â 0.52 mm Structure Reports T = 296 K Online Data collection ISSN 1600-5368 Stowe IPDS 2 diffractometer 16780 measured reflections Absorption correction: integration 2531 independent reflections (X-RED32; Stoe & Cie, 2002) 2225 reflections with I >2(I) 1,3-Bis(3-phenylpropyl)-1H-1,3- Tmin = 0.322, Tmax = 0.408 Rint = 0.055 benzimidazole-2(3H)-selone Refinement 2 2 ˚ À3 R[F >2(F )] = 0.032 Ámax = 0.17 e A a b b 2 ˚ À3 Mehmet Akkurt, *U¨ lku¨ Yılmaz, Hasan Ku¨c¸u¨kbay and wR(F ) = 0.066 Ámin = À0.24 e A Orhan Bu¨yu¨kgu¨ngo¨rc S = 1.07 Absolute structure: Flack (1983), 2531 reflections 1003 Freidel pairs a 128 parameters Flack parameter: 0.004 (12) Department of Physics, Faculty of Sciences, Erciyes University, 38039 Kayseri, H-atom parameters constrained Turkey, bDepartment of Chemistry, Faculty of Arts and Sciences,´ Ino¨nu¨ University, 44280 Malatya, Turkey, and cDepartment of Physics, Faculty of Arts and Sciences, Ondokuz Mayıs University, 55139 Samsun, Turkey Correspondence e-mail: [email protected] Data collection: X-AREA (Stoe & Cie, 2002); cell refinement: X- AREA; data reduction: X-RED32 (Stoe & Cie, 2002); program(s) Received 18 March 2011; accepted 13 April 2011 used to solve structure: SIR97 (Altomare et al., 1999); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008); molecular Key indicators: single-crystal X-ray study; T = 296 K; mean (C–C) = 0.004 A˚; graphics: ORTEP-3 (Farrugia, 1997); software used to prepare R factor = 0.032; wR factor = 0.066; data-to-parameter ratio = 19.8. -

Stereochemistry, Alkyl Halide Substitution (SN1 & SN2)
Chem 261 Assignment & Lecture Outline 3: Stereochemistry, Alkyl Halide Substitution (SN1 & SN2) Read Organic Chemistry, Solomons, Fryle & Snyder 12th Edition (Electronic) • Functional Group List – Learn to recognize – Please see Green Handout – also p 76 of text • Periodic Table – Inside Front Cover - know 1st 10 elements (up through Neon) • Relative Strength of Acids and Bases – Inside Front cover (reference only) • Chapter 5 – Stereochemistry • Chapter 6 –Ionic Reactions: Nucleophilic Substitution of Alkyl Halides Problems: Do Not turn in, answers available in "Study Guide Student Solutions Manual " Solomons, Fryle, Snyder • Chapter 5: 5.1 to 5.15; 5.18 to 5.21; 5.26; 5.28; 5.33a-d; 5.46 • Chapter 6: 6.1 to 6.5; 6.7 to 6.10; 6.13; 6.20; 6.26; 6.27 Lecture Outline # 3 I. Comparison of 2 Structures: Same Molecular Formula ? -> If Yes, Possibly Isomers or Identical Same Arrangement (Sequence) of Groups ? If No -> Structural Isomers If Yes -> Superposable? If Yes -> Identical Structures If No -> Stereoisomers Non-Superposable Mirror Images ? If NO -> Diastereomers If Yes -> Enantiomers II. Chirality and Stereoisomers A. The Concept of Chirality 1. Identification of chiral objects a) achiral = not chiral b) planes of symmetry within a molecule 2. Types of stereoisomers – enantiomers and diastereomers B. Location of stereogenic (chiral) centres – 4 different groups on tetrahedral atom 1. Enantiomers & diastereomers 2. Meso compounds - chiral centers with plane of symmetry within molecule 3. Molecules with more than one chiral centre 4. Recognition of chiral centers in complex molecules - cholesterol - 8 chiral centres Drawing the enantiomer of cholesterol and its potential 255 stereoisomers 5. -

II. Stereochemistry 5
B.Sc.(H) Chemistry Semester - II Core Course - III (CC-III) Organic Chemistry - I II. Stereochemistry 5. Physical and Chemical Properties of Stereoisomers Dr. Rajeev Ranjan University Department of Chemistry Dr. Shyama Prasad Mukherjee University, Ranchi 1 Syllabus & Coverage Syllabus II Stereochemistry: Fischer Projection, Newmann and Sawhorse Projection formulae and their interconversions. Geometrical isomerism: cis–trans and syn-anti isomerism, E/Z notations with Cahn Ingold and Prelog (CIP) rules for determining absolute configuration. Optical Isomerism: Optical Activity, Specific Rotation, Chirality/Asymmetry, Enantiomers, Molecules with two or more chiral-centres, Distereoisomers, Meso structures, Racemic mixture. Resolution of Racemic mixtures. Relative and absolute configuration: D/L and R/S designations. Coverage: 1. Types of Isomers : Comparing Structures 2. Optical Activity 3. Racemic Mixtures : Separation of Racemic Mixtures 4. Enantiomeric Excess and Optical Purity 5. Relative and Absolute Configuration 6. Physical and Chemical Properties of Stereoisomers 2 Stereochemistry Types of Isomers Dr. Rajeev Ranjan 3 Stereochemistry Determining the Relationship Between Two Non-Identical Molecules Dr. Rajeev Ranjan 4 Stereochemistry Comparing Structures: Are the structures connected the same? yes no Are they mirror images? Constitutional Isomers yes no Enantiomers Enantiomers Is there a plane of symmetry? All chiral centers will be opposite between them. yes no Meso Diastereomers superimposable Dr. Rajeev Ranjan 5 Stereochemistry Optical Activity: • The chemical and physical properties of two enantiomers are identical except in their interaction with chiral substances. • The physical property that differs is the behavior when subjected to plane-polarized light ( this physical property is often called an optical property). • Plane-polarized (polarized) light is light that has an electric vector that oscillates in a single plane. -

Amide Activation: an Emerging Tool for Chemoselective Synthesis
Featuring work from the research group of Professor As featured in: Nuno Maulide, University of Vienna, Vienna, Austria Amide activation: an emerging tool for chemoselective synthesis Let them stand out of the crowd – Amide activation enables the chemoselective modification of a large variety of molecules while leaving many other functional groups untouched, making it attractive for the synthesis of sophisticated targets. This issue features a review on this emerging field and its application in total synthesis. See Nuno Maulide et al., Chem. Soc. Rev., 2018, 47, 7899. rsc.li/chem-soc-rev Registered charity number: 207890 Chem Soc Rev View Article Online REVIEW ARTICLE View Journal | View Issue Amide activation: an emerging tool for chemoselective synthesis Cite this: Chem. Soc. Rev., 2018, 47,7899 Daniel Kaiser, Adriano Bauer, Miran Lemmerer and Nuno Maulide * It is textbook knowledge that carboxamides benefit from increased stabilisation of the electrophilic carbonyl carbon when compared to other carbonyl and carboxyl derivatives. This results in a considerably reduced reactivity towards nucleophiles. Accordingly, a perception has been developed of amides as significantly less useful functional handles than their ester and acid chloride counterparts. Received 27th April 2018 However, a significant body of research on the selective activation of amides to achieve powerful DOI: 10.1039/c8cs00335a transformations under mild conditions has emerged over the past decades. This review article aims at placing electrophilic amide activation in both a historical context and in that of natural product rsc.li/chem-soc-rev synthesis, highlighting the synthetic applications and the potential of this approach. Creative Commons Attribution 3.0 Unported Licence. -

And Bis (Imidazoline Selone) Ligands for Visible-Light-Induced Oxidative
Article pubs.acs.org/Organometallics Iridium Complexes Containing Bis(imidazoline thione) and Bis(imidazoline selone) Ligands for Visible-Light-Induced Oxidative Coupling of Benzylamines to Imines † ‡ † † ‡ ‡ Jaewon Jin, Hee-Won Shin, Joon Hyun Park, Ji Hoon Park, Eunchul Kim, Tae Kyu Ahn,*, † † ‡ Do Hyun Ryu,*, and Seung Uk Son*, , † ‡ Department of Chemistry and Department of Energy Science, Sungkyunkwan University, Suwon 440-746, Korea *S Supporting Information ABSTRACT: Novel iridium(III) complexes containing bis- (N-heterocyclic carbene), bis(imidazoline thione) L2, and bis(imidazoline selone) L3 were prepared. The iridium complexes bearing L2 and L3 showed the significant absorption of visible light with maximum intensity at ∼460 nm. Bis(2-(2′-benzothienyl)pyridinato)iridium(III) complexes (Ir-6) with L3 showed excellent ability as a photosensitizer of visible light. Under blue LED irradiation with maximum emission at 460 nm, 0.25 mol % Ir-6 showed 94% conversion of benzylamine for 5 h at room temperature. Through mechanistic studies, it was suggested that the photoinduced oxidative coupling of benzylamine by Ir-6 follows a singlet oxygen pathway. The excellent performance of Ir-6 originated from the efficient visible light absorption at 460 nm and the enhanced triplet state due to the heavy-atom effect of L3. This work shows that bis(imidazoline thione) and bis(imidazoline selone) can be efficient ligands for tuning the optical properties of iridium(III) complexes. ■ INTRODUCTION in photoinduced oxidative coupling of benzylamines to imines Recently, chemical reactions triggered by sustainable energy in the presence of oxygen. sources such as solar energy and the relevant photoactive systems have attracted the attention of scientists.1 Over the past ■ RESULTS AND DISCUSSION decade, iridium(III) complexes bearing the 2-phenylpyridinato Scheme 1 shows the synthetic methods for new iridium 2 (ppy) ligand have shown interesting optical properties. -

Table of Contents
Table of Contents General Co-Chairs Welcome Letter ................................. 2 ACS President Welcome Letter ........................................ 3 Welcome Letter Covington Mayor ................................... 4 Mobile App ....................................................................... 5 Organizing Committee ..................................................... 6 Region Officers ................................................................. 7 General Information ........................................................ 8 Bruker Advertisement ...................................................... 9 Praxair Advertisement ................................................... 10 Advertisement One ........................................................ 11 Commercial Supporters ................................................. 12 Academic Exhibitors ....................................................... 14 Professional Society Supporters .................................... 15 Local Section Donors ...................................................... 16 Meeting at a Glance Wednesday Morning .................... 17 Meeting at a Glance Wednesday Afternoon ................. 18 Meeting at a Glance Thursday Morning ........................ 19 Meeting at a Glance Thursday Afternoon ...................... 20 Meeting at a Glance Friday Morning ............................. 21 Meeting at a Glance Friday Afternoon ........................... 22 Meeting at a Glance Saturday ........................................ 23 ACS Governance -

Enantiomeric Resolution and Absolute Configuration of a Chiral Δ-Lactam
molecules Article Enantiomeric Resolution and Absolute Configuration of a Chiral δ-Lactam, Useful Intermediate for the Synthesis of Bioactive Compounds 1, 1, 1 1 Roberta Listro y, Giacomo Rossino y, Serena Della Volpe , Rita Stabile , Massimo Boiocchi 2 , Lorenzo Malavasi 3, Daniela Rossi 1,* and Simona Collina 1 1 Department of Drug Sciences, University of Pavia, via Taramelli 12, 27100 Pavia, Italy; [email protected] (R.L.); [email protected] (G.R.); [email protected] (S.D.V.); [email protected] (R.S.); [email protected] (S.C.) 2 Centro Grandi Strumenti, University of Pavia, via Bassi 21, 27100 Pavia, Italy; [email protected] 3 Department of Chemistry, University of Pavia, via Taramelli 12, 27100 Pavia, Italy; [email protected] * Correspondence: [email protected] These authors contributed equally to this work. y Academic Editor: Józef Drabowicz Received: 2 December 2020; Accepted: 18 December 2020; Published: 19 December 2020 Abstract: During the past several years, the frequency of discovery of new molecular entities based on γ- or δ-lactam scaffolds has increased continuously. Most of them are characterized by the presence of at least one chiral center. Herein, we present the preparation, isolation and the absolute configuration assignment of enantiomeric 2-(4-bromophenyl)-1-isobutyl-6-oxopiperidin-3-carboxylic acid (trans-1). For the preparation of racemic trans-1, the Castagnoli-Cushman reaction was employed. (Semi)-preparative enantioselective HPLC allowed to obtain enantiomerically pure trans-1 whose absolute configuration was assigned by X-ray diffractometry. Compound (+)-(2R,3R)-1 represents a reference compound for the configurational study of structurally related lactams. -

Alkyl Halides
Alkyl Halides Alkyl halides are a class of compounds where a halogen atom or atoms are bound to an sp3 orbital of an alkyl group. CHCl3 (Chloroform: organic solvent) CF2Cl2 (Freon-12: refrigerant CFC) CF3CHClBr (Halothane: anesthetic) Halogen atoms are more electronegative than carbon atoms, and so the C-Hal bond is polarized. The C-Hal (often written C-X) bond is polarized in such a way that there is partial positive charge on the carbon and partial negative charge on the halogen. Ch06 Alkyl Halides (landscape).docx Page 1 Dipole moment Electronegativities decrease in the order of: F > Cl > Br > I Carbon-halogen bond lengths increase in the order of: C-F < C-Cl < C-Br < C-I Bond Dipole Moments decrease in the order of: C-Cl > C-F > C-Br > C-I = 1.56D 1.51D 1.48D 1.29D Typically the chemistry of alkyl halides is dominated by this effect, and usually results in the C-X bond being broken (either in a substitution or elimination process). This reactivity makes alkyl halides useful chemical reagents. Ch06 Alkyl Halides (landscape).docx Page 2 Nomenclature According to IUPAC, alkyl halides are treated as alkanes with a halogen (Halo-) substituent. The halogen prefixes are Fluoro-, Chloro-, Bromo- and Iodo-. Examples: Often compounds of CH2X2 type are called methylene halides. (CH2Cl2 is methylene chloride). CHX3 type compounds are called haloforms. (CHI3 is iodoform). CX4 type compounds are called carbon tetrahalides. (CF4 is carbon tetrafluoride). Alkyl halides can be primary (1°), secondary (2°) or tertiary (3°). Other types: A geminal (gem) dihalide has two halogens on the same carbon. -

Copyrighted Material
CONTENTS Preface xxi Acknowledgments xxiii 1. Introduction 1 1.1. Chiral Derivatizing Agents 1 1.2. Chiral Solvating Agents 2 1.3. Overview of Chiral Reagents and Methodologies 4 1.4. Future Prospects 6 2. Aryl-Containing Carboxylic Acids 8 2.1. Introduction 8 2.2. a-Methoxy-a-trifluoromethylphenylacetic Acid (MTPA–Mosher’s Reagent) 11 2.2.1. Analysis of Secondary Alcohols 13 2.2.2. Analysis of Secondary Diols and Polyols 19 2.2.3.COPYRIGHTED Analysis of Primary Alcohols MATERIAL 25 2.2.4. Analysis of Tertiary Alcohols 26 2.2.5. Analysis of Secondary Amines 26 2.2.6. Analysis of Primary Amines 28 2.2.7. Use as a Chiral Solvating Agent 29 2.2.8. Use of MTPA Derivatives with Paramagnetic Lanthanide Chelates 30 2.2.9. Use of MTPA Derivatives with Diamagnetic Lanthanide Chelates 34 vii viii CONTENTS 2.2.10. Preparation of MTPA Derivatives 35 2.2.11. Liquid Chromatography–NMR Spectroscopy of MTPA Derivatives 35 2.2.12. Database Methods with MTPA 35 2.3. a-Methoxyphenylacetic Acid (O-methyl Mandelic Acid-MPA) 38 2.3.1. Analysis of Secondary Alcohols 39 2.3.2. Analysis of Diols 42 2.3.3. Analysis of Primary Alcohols 43 2.3.4. Analysis of Amines 44 2.3.5. Analysis of Sulfoxides 45 2.3.6. Variable-temperature Method for Assigning Absolute Stereochemistry 46 2.2.7. Barium(II) Method for Assigning Absolute Stereochemistry 47 2.3.8. Use of MPA Derivatives with Lanthanide Chelates 48 2.3.9. Use as a Chiral Solvating Agent 49 2.3.10. -

The Chemistry of Tertiary Amides and Related Compounds
Louisiana State University LSU Digital Commons LSU Historical Dissertations and Theses Graduate School 1967 The heC mistry of Tertiary Amides and Related Compounds. Kalil Phillip Ieyoub Louisiana State University and Agricultural & Mechanical College Follow this and additional works at: https://digitalcommons.lsu.edu/gradschool_disstheses Recommended Citation Ieyoub, Kalil Phillip, "The heC mistry of Tertiary Amides and Related Compounds." (1967). LSU Historical Dissertations and Theses. 1252. https://digitalcommons.lsu.edu/gradschool_disstheses/1252 This Dissertation is brought to you for free and open access by the Graduate School at LSU Digital Commons. It has been accepted for inclusion in LSU Historical Dissertations and Theses by an authorized administrator of LSU Digital Commons. For more information, please contact [email protected]. This dissertation has been microfilmed exactly as received 67-8783 IEYOUB, Kalil Phillip, 1935- THE CHEMISTRY OF TERTIARY AMIDES AND RELATED COMPOUNDS. Louisiana State University and Agricultural and Mechanical College, Ph.D., 1967 Chemistry, organic University Microfilms, Inc., Ann Arbor, Michigan Reproduced with permission of the copyright owner. Further reproduction prohibited without permission. THE CHEMISTRY OE TERTIARY AMIDES AND RELATED COMPOUNDS A Dissertation Submitted to the Graduate Faculty of the Louisiana State University and Agricultural and Mechanical College in partial fulfillment of the requirements for the degree of Doctor of Philosophy in The Department of Chemistry by K alil P h illip leyoub B.S., McNe.ese State College, 195^ M.S., Louisiana State University, I 965 January, 1967 Reproduced with permission of the copyright owner. Further reproduction prohibited without permission. ACKNOWLEDGMENT The author wishes to express his sincere appreciation for the guidance given him by Dr. -

II. Nomenclature Rules for Alkenes 1. the Parent Name Will Be the Longest
1 Lecture 9 II. Nomenclature Rules For Alkenes 1. The parent name will be the longest carbon chain that contains both carbons of the double bond. Drop the -ane suffix of the alkane name and add the –ene suffix. Never name the double bond as a prefix. If a double bond is present, you have an alkene, not an alkane. alkane + -ene = alkene 2. Begin numbering the chain at the end nearest the double bond. Always number through the double bond and identify its position in the longest chain with the lower number. In the older IUPAC rules the number for the double bond was placed in front of the stem name with a hyphen. Under the newer rules, the number for the double bond is placed right in front of “ene”, with hyphens. We will use the newer rules for specifying the location of pi bonds. 1 2 3456 H3CCHCH CH 2 CH2 CH3 hex-2-ene (newer rules) 2-hexene (older rules) 3. Indicate the position of any substituent group by the number of the carbon atom in the parent (longest) chain to which it is attached. CH 1 2 345 3 H3CCHCHCHCH2 CH CH3 6 7 CH3 Numbering is determined by the double bond, not the branches, because the double bond has 5,6-dimethylhept-3-ene (newer rules) higher priority than any alkyl branch. 5,6-dimethyl-3-heptene (older rules) 4. Number cycloalkenes so that the double bond is 1,2 (number through the double bond). Number in the direction about the ring so that the lowest number is used at the first point of difference. -
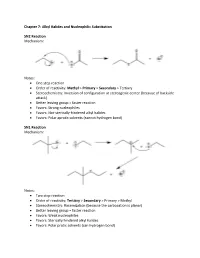
Alkyl Halides and Nucleophilic Substitution SN2 Reaction
Chapter 7: Alkyl Halides and Nucleophilic Substitution SN2 Reaction Mechanism: Notes: • One step reaction • Order of reactivity: Methyl > Primary > Secondary > Tertiary • Stereochemistry: Inversion of configuration at stereogenic center (because of backside attack) • Better leaving group = faster reaction • Favors: Strong nucleophiles • Favors: Not-sterically-hindered alkyl halides • Favors: Polar aprotic solvents (cannot hydrogen bond) SN1 Reaction Mechanism: Notes: • Two step reaction • Order of reactivity: Tertiary > Secondary > Primary > Methyl • Stereochemistry: Racemization (because the carbocation is planar) • Better leaving group = faster reaction • Favors: Weak nucleophiles • Favors: Sterically hindered alkyl halides • Favors: Polar protic solvents (can hydrogen bond) Important Trends Chapter 8: Alkyl Halides and Elimination Reactions E2 Reaction Mechanism: Notes: • One step reaction • Order of reactivity: Tertiary > Secondary > Primary • Stereochemistry: antiperiplanar arrangement of H and X • Better leaving group = faster reaction • Favors: Polar aprotic solvents, strong bases • Products follow Zaitsev rule (more substituted alkene is the major product) E1 Reaction Mechanism: Notes: • Two step reaction • Order of reactivity: Tertiary > Secondary > Primary • Stereochemistry: Trigonal planar carbocation intermediate • Better leaving group = faster reaction • Favors: Polar protic solvents, weak bases • Products follow Zaitsev rule Chapter 9: Alcohols, Ethers, and Epoxides Preparation of Alcohols Mechanism: Notes: • SN2 mechanism