Nitro and Cyno Compounds Preparation of Nitro Compounds
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Nitro Compounds As Oxidizing Agents
Class Book _______ Accession 1-f c6-- 111 Vol. ______ MORRISON . LIBRARY OF THE Municipal University of Wichita WICHITA. KANSAS THE IVERSI~Y ·o v !CHIT. NIT O COMPOUH ]).) 0 ID! ING AGENTw . SUBMITTED TO THE G.1. IDATE FACULTY IN C DI CY FOR THE ·n~G. OF TERO TS ) ) :l)-, .l)) )) ) ! ~ ):> ) ) ) .) ) .). J ) ,, ) ) ..) )) )) .) } ::> ) ) ) ) J ) J ) J ) .) ) .) ) .) ) . ) , ) ) ) ) ..) ) ) ) ) } ) J).) ' .J ) "):) ) ) .) .) ) ::> .) J ) ) ' J .) J ) ) s JUN t J.~32 Acknov~e gment is made to Professor ·orth A. Fletcher for his direction and assistance in the con uctance of this study. (ii) (ii) T BLE ·0] 1 COMTiilllT :e G.J.:J CKU ONLE DGlJCNT • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • . ii LIT OFT BLE • • • • • • • • • • • • • • • • • • • • • • • • , • • • , , • • • i V LI T OF FIGUl ~ ••••••••••••••••••••••• ' •••••••••• V IN Tl ODUCTI ON ••••••••••••••••••• , •••••••••••••••• l ~ pv. 11JI:E:t.i T L •· • • • • • • • • • • , • , •· • , , • • , • • • • • • • • • • • • • • • t ]? T B DFEECT OF TE1ft.PER TUHE •••• • • • • • • • • • • • .Id • C ffi.,' • y •••• •••••••••••• ............ •' • • • • • •••••• 13 LI 11: J:RE: CI TE'D. • • • • ••• , • • • • • • • • • • • • • • • , • • • • • •• 14 (iii) LI T O · BLE Table page I• • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • .-:. • • • • • • 5 I I •• .. ................ ··~. ·~ ·· .....•..•........••.• 7 III. • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • 8 rv~.. J ••••••••••••••••••••••••••••••• .•••••••••••• -

Nitroso and Nitro Compounds 11/22/2014 Part 1
Hai Dao Baran Group Meeting Nitroso and Nitro Compounds 11/22/2014 Part 1. Introduction Nitro Compounds O D(Kcal/mol) d (Å) NO NO+ Ph NO Ph N cellular signaling 2 N O N O OH CH3−NO 40 1.48 molecule in mammals a nitro compound a nitronic acid nitric oxide b.p = 100 oC (8 mm) o CH3−NO2 57 1.47 nitrosonium m.p = 84 C ion (pKa = 2−6) CH3−NH2 79 1.47 IR: υ(N=O): 1621-1539 cm-1 CH3−I 56 Nitro group is an EWG (both −I and −M) Reaction Modes Nitro group is a "sink" of electron Nitroso vs. olefin: e Diels-Alder reaction: as dienophiles Nu O NO − NO Ene reaction 3 2 2 NO + N R h 2 O e Cope rearrangement υ O O Nu R2 N N N R1 N Nitroso vs. carbonyl R1 O O O O O N O O hυ Nucleophilic addition [O] N R2 R O O R3 Other reaction modes nitrite Radical addition high temp low temp nitrolium EWG [H] ion brown color less ion Redox reaction Photochemical reaction Nitroso Compounds (C-Nitroso Compounds) R2 R1 O R3 R1 Synthesis of C-Nitroso Compounds 2 O R1 R 2 N R3 3 R 3 N R N R N 3 + R2 2 R N O With NO sources: NaNO2/HCl, NOBF4, NOCl, NOSbF6, RONO... 1 R O R R1 O Substitution trans-dimer monomer: blue color cis-dimer colorless colorless R R NOBF OH 4 - R = OH, OMe, Me, NR2, NHR N R2 R3 = H or NaNO /HCl - para-selectivity ΔG = 10 Kcal mol-1 Me 2 Me R1 NO oxime R rate determining step Blue color: n π∗ absorption band 630-790 nm IR: υ(N=O): 1621-1539 cm-1, dimer υ(N−O): 1300 (cis), 1200 (trans) cm-1 + 1 Me H NMR (α-C-H) δ = 4 ppm: nitroso is an EWG ON H 3 Kochi et al. -

Electrochemistry and Photoredox Catalysis: a Comparative Evaluation in Organic Synthesis
molecules Review Electrochemistry and Photoredox Catalysis: A Comparative Evaluation in Organic Synthesis Rik H. Verschueren and Wim M. De Borggraeve * Department of Chemistry, Molecular Design and Synthesis, KU Leuven, Celestijnenlaan 200F, box 2404, 3001 Leuven, Belgium; [email protected] * Correspondence: [email protected]; Tel.: +32-16-32-7693 Received: 30 March 2019; Accepted: 23 May 2019; Published: 5 June 2019 Abstract: This review provides an overview of synthetic transformations that have been performed by both electro- and photoredox catalysis. Both toolboxes are evaluated and compared in their ability to enable said transformations. Analogies and distinctions are formulated to obtain a better understanding in both research areas. This knowledge can be used to conceptualize new methodological strategies for either of both approaches starting from the other. It was attempted to extract key components that can be used as guidelines to refine, complement and innovate these two disciplines of organic synthesis. Keywords: electrosynthesis; electrocatalysis; photocatalysis; photochemistry; electron transfer; redox catalysis; radical chemistry; organic synthesis; green chemistry 1. Introduction Both electrochemistry as well as photoredox catalysis have gone through a recent renaissance, bringing forth a whole range of both improved and new transformations previously thought impossible. In their growth, inspiration was found in older established radical chemistry, as well as from cross-pollination between the two toolboxes. In scientific discussion, photoredox catalysis and electrochemistry are often mentioned alongside each other. Nonetheless, no review has attempted a comparative evaluation of both fields in organic synthesis. Both research areas use electrons as reagents to generate open-shell radical intermediates. Because of the similar modes of action, many transformations have been translated from electrochemical to photoredox methodology and vice versa. -

Conjugate Additions of Nitroalkanes to Electron-Poor Alkenes: Recent Results
Chem. Rev. 2005, 105, 933−971 933 Conjugate Additions of Nitroalkanes to Electron-Poor Alkenes: Recent Results Roberto Ballini,* Giovanna Bosica, Dennis Fiorini, Alessandro Palmieri, and Marino Petrini* Dipartimento di Scienze Chimiche, Universita` di Camerino, via S. Agostino, 1, I-62032 Camerino, Italy Received September 30, 2004 Contents Conjugate additions using highly stabilized carban- ions are still of interest since a growing number of 1. Introduction 933 these procedures can be carried out in environmen- 2. General Aspects of the Conjugate Addition of 934 tally benign solvents such as water and using cata- Nitroalkanes lytic amounts of the basic promoter. In addition, the 2.1. Multiple Additions 934 achievement of diastereo- and enantioselective pro- 2.2. Basic Catalysts 935 cesses is no longer an exclusive domain of highly 3. New Basic Catalysts for the Conjugate Addition 936 reactive carbanionic systems working in carefully 4. Diastereoselective Conjugate Additions 936 controlled conditions3 but can be nowadays conducted 4.1. Intermolecular Additions 936 even at room temperature using easily available 4.2. Intramolecular Additions 948 substrates and suitable base/solvent combinations. 5. Asymmetric Conjugate Additions Promoted by 949 Nitroalkanes are a valuable source of stabilized Chiral Catalysis carbanions since the high electron-withdrawing power 6. Conjugate Addition−Elimination Reactions 953 of the nitro group provides an outstanding enhance- R 7. Synthetic Applications 957 ment of the hydrogen acidity at the -position (cf. pka ) 4-8 7.1. Pyrrolidines and Derivatives 957 MeNO2 10). Nitronate anions 2 that can be generated from nitroalkanes 1 using a wide range of 7.2. Lactones and Oxygenated Heterocycles 960 bases act as carbon nucleophiles with common elec- 7.3. -

UNITED STATES PATENT Orricsg. 2,623,904 Nrrao ALDEHYDES and Preraaarion TPEREGF Curtis W
Patented Dec. 30, ‘1952 2,623.04 UNITED STATES PATENT orricsg. 2,623,904 nrrao ALDEHYDES AND PRErAaArioN _ TPEREGF Curtis W. Smith, ‘Berke ley, Calif., assignor to Shell Development Company, San Francisco, Calif., a corporation of Delaware No Drawing. Application December 3, 1948, Serial No. 63,455 13 Claims. (Cl. 260-601) substituent group is directly linked to an- all This invention relates to organic compounds phatic carbon atom to which there is also direct and to a process for the preparation of organic ly attached an atom of hydrogen, nitro-aldehydes compounds. rather than nitro-alcohols, are produced. ' More particularly, the invention relates to The alpha-methylidene aldehydes are those nitrmaldehydes and to a process for the prepara aldehydes which have directly linked to the car tion of nitro-aldehydes. The invention also re bon atom in the alpha position relative to the lates to a new and unexpected reaction of ‘un formyl group a methylidene radical (CI-I2=),. saturated aldehydes with organic nitro com Thus, they are the alpha, beta-ole?nic aldehydes pounds whereby the nitro-aldehydes of the in in which the remaining valences of thecarbon vention may be prepared. atom in the beta position are satis?ed byatoms It has been discovered that nitro-aldehydes of hydrogen. The alpha-methylidene aldehydes may be prepared by condensing alpha-methyli may also be described by means of the formula dene aldehydes with nitro-substituted compounds wherein a nitro substituent group is directly linked to an aliphatic carbon atom to which there is also directly attached an atom of hydrogen. -

First and Efficient Method for Reduction of Aliphatic and Aromatic Nitro Compounds with Zinc Borohydride As Pyridine Zinc Tetrah
Journal of the Chinese Chemical Society, 2003, 50, 267-271 267 First and Efficient Method for Reduction of Aliphatic and Aromatic Nitro Compounds with Zinc Borohydride as Pyridine Zinc Tetrahydroborato Complex: A New Stable Ligand-Metal Borohydride Behzad Zeynizadeh* and Karam Zahmatkesh Chemistry Department, Faculty of Sciences, Urmia University, Urmia 57159, Iran Pyridine zinc tetrahydroborate, [(Py)Zn(BH4)2], as a new stable ligand-metal borohydride, is prepared quantitatively by complexation of 1:1 zinc borohydride and pyridine at room temperature. This reagent effi- ciently reduces different aromatic and aliphatic nitro compounds to their primary amines in refluxing THF. In addition, the reduction shows chemoselectivity for aliphatic nitro compounds over the aromatic nitro com- pounds. Keywords: Reduction; Zinc borohydride; Nitro compound; Chemoselectivity; Amine. INTRODUCTION been reviewed. These successes prompted us to prepare 13 [(Py)Zn(BH4)2] and investigate its reducing ability. In our For over forty years, sodium borohydride has been study, we also observed that this reagent efficiently reduces widely recognized as the reagent of choice for the reduction aliphatic and aromatic nitro compounds to their correspond- of carbonyl compounds and acid chlorides in protic solvents.1 ing primary amines in refluxing THF (Scheme I). Over the past decades, the utility of sodium borohydride has been greatly expanded and different techniques and modifi- Scheme I cations have been developed for it to reduce organofunctional groups in various -

Organic Chemistry Masterclasses
Organic Chemistry Masterclasses By S. Ranganathan All rights reserved. No parts of this publication may be reproduced, stored in a retrieval system, or transmitted, in any form or by any means, electronic, mechanical, photocopy- ing, recording, or otherwise, without prior permission of the publisher. © Indian Academy of Sciences Published by Indian Academy of Sciences 2 Foreword The Masterclass series of eBooks published by the Indian Academy of Sciences, Bengaluru brings together pedagogical articles on single broad topics reproduced from Resonance, Journal of Science Education that is published monthly by the Academy since January 1996. Primarily directed at students and teachers at the undergraduate level, the journal has brought out a wide spectrum of articles in a range of scientific disciplines. Articles in the journal are written in a style that makes them appealing to readers from diverse backgrounds, and in addition, they provide a useful source of instruction that is not always available in textbooks. It is fitting that the first book in this series,Organic Chemistry Masterclasses, is by a legendary teacher, Professor Subramania Ranganathan, highly acclaimed and distinguished Organic Chemist. Ranga, as he is fondly known to generations of students at the IIT Kanpur where he taught for over three decades, has been a regular contributor to Resonance and his articles enjoy wide popularity among the journal’s readership. Indeed his first contribution toResonance was in the very first issue of the journal. We are also delighted that this collection of over fifteen articles, reformatted as an eBook will mark his 80th birthday. Those of us who were fortunate to have learned some of these topics as he was devising ways of teaching them will be very pleased that some of Ranga’s masterly instructions can reach a much wider audience through this means. -

Electrolytic Reduction of Certain Organic Nitro Compounds And
AN ABSTRACT OF THE THESIS OF Frank Warren Thomas M.S. Chenilstry --------------- forthe- ----- In ----------- (Name) (Degree) (Major) Date Thesis presented _.L_° ______ Tit le _' Nitro Coniounds and HeterocZcl1cNitroenBases1v. Redacted for privacy Abstract Approved: (Major Professor) The reduction by eleotrochemical methods of certain nitro oompounds and heterocyclic nitrocyn bases occurs readily to produce a wide variety of substances. Experimental evidence, as yet unpublished, Indicates that certain reduced products may have a definite commercial value. The purpose of this research is to demonstrate that electrochemical methods can be used to reduce the types of organic compounds selected for this work. The elementary laws of electrochemistry were placed on a firm basis by the labors of Michael Faraday in 134. Since that time Lob, Brockman, Creihton and Koehier have made S available various texts summarizing the electrochemical data of several decades. The addItion or substitution of hydroen to organic compounds is of sufficient commercial importance to justify the devel:pment of special metods of hydrogenation. - The development of an experimental technique must reccde the development of any actual commercial method. The methods developed in this v:ork show the extent of reduction of the particular compounds considere under definite conditions. These conditions and results have been tabulated in the Thesis entitled "Reduction of certain .romatic Nitro Compounds and Heterocyclic Nitrogen Bases", submited to Oreton State College by Frank -

A Simple Method for Analysis of Nitrobenzene in Aniline"
Indian Journal of Chemistry Vol. 21A, March 1982, pp. 279-281 A Simple Method for Analysis of Nitrobenzene In Aniline" SUKUMAR MAITI- Materials Science Centre, Indian Institute of Technology, Kharagpur 721 302 and B. H. PATEL & (Miss) BHAVNA M. PANDYA Deepak Nitrite Ltd, Baroda 391 340 Received 7 June 1980; revised 19 May 1981; accepted 3 July 1981 A simple and sensitive method for the detennination of nitrobenzene in aniline and other aromatic nitro com- pounds has been developed. The method is based on the colour formation when nitrobenzene reacts with acetone- KOH reagent. The colour thus obtained obeys Lambert-Beer's law and therefore offers an elegant and simple colori- metric method for the determination of trace amount of nitrobenzene in aniline. The colour reaction is very specific; only ortho and para-positions to nitro groups are reactive. A mechanism of the reaction has been suggested. perusal of the literature reveals that so far no volume (5-10 ml). The ether was completely removed simple and sensitive method is available for by adding acetone followed by evaporation. The A the determination of nitro benzene in com- concentrated extract was tested as usual. By this mercial samples of aniline. Acetone/potassium hy- enrichment technique nitrobenzene present in the droxide produces colour with di- and poly-nitro aro- order of 1-5 ppm has been successfully estimated. matic compounds but not with mononitro compounds (Janovsky reaction'). We have re-examined this Results and Discussion reaction in the case of mononitro aromatic com- Lambert and Beer's law is found to be obeyed pounds, particularly of nitrobenzene in aniline, in the concentration range 0-1,000 ppm of nitro- and have been able to develop a sensitive and simple benzene in aniline. -

Reductive Metabolism of Aromatic Nitro Compounds Including Carcinogens by Rabbit Liver Preparations1
[CANCER RESEARCH 46, 1089-1093, March 1986] Reductive Metabolism of Aromatic Nitro Compounds Including Carcinogens by Rabbit Liver Preparations1 Kiyoshi Tatsumi,2 Shigeyuki Kitamura, and Noriko Narai3 Institute of Pharmaceutical Sciences, Hiroshima University School of Medicine, 1-2-3, Kasumi, Minami-ku, Hiroshima 734, Japan ABSTRACT somal and cytosolic fractions of mammalian livers (15, 18, 20, 22-24). Previous studies showed that a cytochrome P-450 sys Reductive metabolism of aromatic nitro compounds was ex tem (23, 25) and xanthine oxidase (23) are involved in the amined with rabbit liver preparations. Under anaerobic condi reduction of nitrobenzene orp-nitrobenzoic acid catalyzed by rat tions, carcinogenic 2-nitrofluorene, 4-nitrobiphenyl, and 1-nitro- liver microsomes and cytosol, respectively. In a preliminary com naphthalene were reduced to the corresponding hydroxylamines munication (18), recently, we demonstrated that a microsomal and amines, whereas the carcinogenic 1-nitropyrene was re cytochrome P-450 system and cytosolic aldehyde oxidase of duced only to the corresponding amine by liver cytosol in the rabbit liver catalyze the reduction of 1-nitropyrene and 2-nitroflu presence of 2-hydroxypyrimidine, an electron donor of aldehyde orene to the corresponding amines. More recently, the partici oxidase. These metabolites were identified unequivocally by pation of cytochrome P-450 in nitroreduction was further con comparing their mass spectra and thin-layer Chromatographie firmed with a reconstituted cytochrome P-450 system from rat behaviors with those of the authentic samples. liver microsomes (20). Both liver microsomes and cytosol catalyzed the reduction of The present study shows the first example of enzymatic these aromatic nitro compounds in varying degrees. -
Thèse Toward the Total Synthesis of Bbiq Alkaloids
THÈSE PRÉSENTÉE A L’UNIVERSITÉ DE BORDEAUX ÉCOLE DOCTORALE DES SCIENCES CHIMIQUES POUR L’OBTENTION DU GRADE DE DOCTEUR SPÉCIALITÉ : CHIMIE ORGANIQUE Par Obinna Uchechukwu OSUJI TOWARD THE TOTAL SYNTHESIS OF BBIQ ALKALOIDS: THE TRILOBINE Directeurs de thèse : Frédéric CANTAGREL Eric FOUQUET Soutenue le: 19 Novembre 2020 Jury: M. Yannick LANDAIS Professeur, Université de Bordeaux Président Mme. Camille OGER Maître de Conférences, Université de Montpellier Rapportrice M. Laurent ELKAIM Directeur de Recherche-ENSTA Rapporteur Mme. Paola ARIMONDO Directrice de Recherche-Institut Pasteur Examinatrice M. Frédéric CANTAGREL Ingénieur de recherche, CNRS Directeur de thèse M. Eric FOUQUET Professeur, Université de Bordeaux Directeur de thèse 1 DEDICATION This research work is dedicated to Chimamanda, Munachisom, and Chibuikem with special thanks and love to Chizoba Obinna Osuji, wife, friend, and companion. And to the members of my family; for their prayers, unconditional love, and moral support, which has allowed me to complete this great landmark in my career. 2 ACKNOWLEDGEMENT This work is the result of a lifetime of learning and development from many mentors, teachers, supporters, advisors, counsellors, friends and family; who invested their time, energy and interest in my life. For this, I am eternally grateful. No achievement in life is without the help of many individuals known and unknown, who contributed to my life and I owe any measure of success to the array of inputs from them all. Here are just a few who made this work possible. To my dear parents, Sir Chukwuemeka and Lady Adaugo Osuji; and my ever interested, encouraging and enthusiastic siblings. You were always keen on my well-being, and the progress of my study in France. -
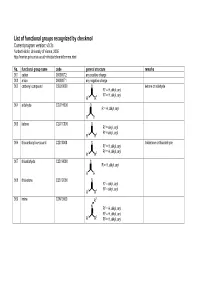
List of Functional Groups Recognized by Checkmol
List of functional groups recognized by checkmol Current program version: v0.3c Norbert Haider, University of Vienna, 2005 http://merian.pch.univie.ac.at/~nhaider/cheminf/cmmm.html No. functional group name code general structure remarks 001 cation 000000T2 any positive charge 002 anion 000000T1 any negative charge 003 carbonyl compound C2O10000 O ketone or aldehyde R1 = H, alkyl, aryl 2 1 2 R = H, alkyl, aryl R R 004 aldehyde C2O1H000 O R = H, alkyl, aryl R H 005 ketone C2O1C000 O R1 = alkyl, aryl 2 1 2 R = alkyl, aryl R R 006 thiocarbonyl compound C2S10000 S thioketone or thioaldehyde R1 = H, alkyl, aryl 2 1 2 R = H, alkyl, aryl R R 007 thioaldehyde C2S1H000 S R = H, alkyl, aryl R H 008 thioketone C2S1C000 S R1 = alkyl, aryl 2 1 2 R = alkyl, aryl R R 009 imine C2N10000 R3 N R1 = H, alkyl, aryl 2 1 2 R = H, alkyl, aryl R R 3 R = H, alkyl, aryl 010 hydrazone C2N1N000 R3 N 1 R4 R = H, alkyl, aryl N R2 = H, alkyl, aryl R3 = H, alkyl, aryl 1 2 R4 = H, alkyl, aryl R R 011 semicarbazone C2NNC4ON R4 O N R5 1 N 3 R = H, alkyl, aryl R 2 N R = H, alkyl, aryl R3 = H, alkyl, aryl 4 1 2 R = H, alkyl, aryl, R R 5 R = H, alkyl, aryl 012 thiosemicarbazone C2NNC4SN R4 S N R5 1 N 3 R = H, alkyl, aryl R 2 N R = H, alkyl, aryl R3 = H, alkyl, aryl 4 1 2 R = H, alkyl, aryl, R R 5 R = H, alkyl, aryl 013 oxime C2N1OH00 OH N R1 = H, alkyl, aryl 2 1 2 R = H, alkyl, aryl R R 014 oxime ether C2N1OC00 OR3 N R1 = H, alkyl, aryl 2 1 2 R = H, alkyl, aryl R R 3 R = alkyl, aryl 015 ketene C3OC0000 O R1 = H, alkyl, aryl 2 1 2 R = H, alkyl, aryl R R 016 ketene acetal derivative