New Pediatric Patient Information Packet
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Surgical Treatment of Snoring and Obstructive Sleep Apnea Syndrome
Medical Policy Surgical Treatment of Snoring and Obstructive Sleep Apnea Syndrome Table of Contents Policy: Commercial Coding Information Information Pertaining to All Policies Policy: Medicare Description References Authorization Information Policy History Policy Number: 130 BCBSA Reference Number: 7.01.101 Related Policies None Policy Commercial Members: Managed Care (HMO and POS), PPO, and Indemnity Medicare HMO BlueSM and Medicare PPO BlueSM Members Uvulopalatopharyngoplasty (UPPP) may be MEDICALLY NECESSARY for the treatment of clinically significant obstructive sleep apnea syndrome (OSAS) in appropriately selected adult patients who have failed an adequate trial of continuous positive airway pressure (CPAP) or failed an adequate trial of an oral appliance (OA). Clinically significant OSA is defined as those patients who have: Apnea/hypopnea Index (AHI) or Respiratory Disturbance Index (RDI) 15 or more events per hour, or AHI or RDI 5 or more events and 14 or less events per hour with documented symptoms of excessive daytime sleepiness, impaired cognition, mood disorders or insomnia, or documented hypertension, ischemic heart disease, or history of stroke. Hyoid suspension, surgical modification of the tongue, and/or maxillofacial surgery, including mandibular- maxillary advancement (MMA), may be MEDICALLY NECESSARY in appropriately selected adult patients with clinically significant OSA and objective documentation of hypopharyngeal obstruction who have failed an adequate trial of continuous positive airway pressure (CPAP) or failed an adequate trial of an oral appliance (OA). Clinically significant OSA is defined as those patients who have: AHI or RDI 15 or more events per hour, or AHI or RDI 5 or more events and 14 or less events per hour with documented symptoms of excessive daytime sleepiness, impaired cognition, mood disorders or insomnia, or documented hypertension, ischemic heart disease, or history of stroke. -
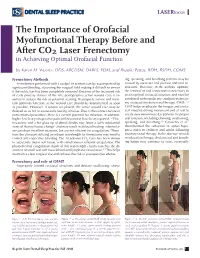
The Importance of Orofacial Myofunctional Therapy Before and After CO2 Laser Frenectomy in Achieving Optimal Orofacial Function
LASERfocus The Importance of Orofacial Myofunctional Therapy Before and After CO2 Laser Frenectomy in Achieving Optimal Orofacial Function by Karen M. Wuertz, DDS, ABCDSM, DABLS, FOM, and Brooke Pettus, RDH, BSDH, COMS Frenectomy Methods ing, speaking, and breathing patterns may be Frenotomies performed with a scalpel or scissors can be accompanied by caused by incorrect oral posture and oral re- significant bleeding, obscuring the surgical field making it difficult to ensure strictions. Therefore, in the authors’ opinion, if the restriction has been completely removed. Because of the increased risk the removal of oral restrictions is necessary to of early primary closure of the site, postoperative active wound care is es- attain optimal orofacial function, and must be sential to reduce the risk of potential scarring. To properly restore and main- combined with regular pre- and post-frenecto- tain optimum function, active wound care should be implemented as soon my orofacial myofunctional therapy (OMT).1,4 as possible. However, if sutures are placed, the active wound care may be OMT helps re-educate the tongue and orofa- delayed so as not to cause early tearing of tissue. Due to the contact nature of cial muscles during movement and at rest to conventional procedure, there is a certain potential for infection; in addition, create new neuromuscular patterns for proper higher levels of postoperative pain and discomfort have been reported.1,2 Elec- oral function, including chewing, swallowing, trocautery and a hot glass tip of dental diodes may leave a fairly substantial speaking, and breathing.5,6 Camacho et al.7 zone of thermal tissue change3 and may result in delayed healing. -

Read Full Article
PEDIATRIC/CRANIOFACIAL Pharyngeal Flap Outcomes in Nonsyndromic Children with Repaired Cleft Palate and Velopharyngeal Insufficiency Stephen R. Sullivan, M.D., Background: Velopharyngeal insufficiency occurs in 5 to 20 percent of children M.P.H. following repair of a cleft palate. The pharyngeal flap is the traditional secondary Eileen M. Marrinan, M.S., procedure for correcting velopharyngeal insufficiency; however, because of M.P.H. perceived complications, alternative techniques have become popular. The John B. Mulliken, M.D. authors’ purpose was to assess a single surgeon’s long-term experience with a Boston, Mass.; and Syracuse, N.Y. tailored superiorly based pharyngeal flap to correct velopharyngeal insufficiency in nonsyndromic patients with a repaired cleft palate. Methods: The authors reviewed the records of all children who underwent a pharyngeal flap performed by the senior author (J.B.M.) between 1981 and 2008. The authors evaluated age of repair, perceptual speech outcome, need for a secondary operation, and complications. Success was defined as normal or borderline sufficient velopharyngeal function. Failure was defined as borderline insufficiency or severe velopharyngeal insufficiency with recommendation for another procedure. Results: The authors identified 104 nonsyndromic patients who required a pharyngeal flap following cleft palate repair. The mean age at pharyngeal flap surgery was 8.6 Ϯ 4.9 years. Postoperative speech results were available for 79 patients. Operative success with normal or borderline sufficient velopharyngeal function was achieved in 77 patients (97 percent). Obstructive sleep apnea was documented in two patients. Conclusion: The tailored superiorly based pharyngeal flap is highly successful in correcting velopharyngeal insufficiency, with a low risk of complication, in non- syndromic patients with repaired cleft palate. -

Core Curriculum for Surgical Technology Sixth Edition
Core Curriculum for Surgical Technology Sixth Edition Core Curriculum 6.indd 1 11/17/10 11:51 PM TABLE OF CONTENTS I. Healthcare sciences A. Anatomy and physiology 7 B. Pharmacology and anesthesia 37 C. Medical terminology 49 D. Microbiology 63 E. Pathophysiology 71 II. Technological sciences A. Electricity 85 B. Information technology 86 C. Robotics 88 III. Patient care concepts A. Biopsychosocial needs of the patient 91 B. Death and dying 92 IV. Surgical technology A. Preoperative 1. Non-sterile a. Attire 97 b. Preoperative physical preparation of the patient 98 c. tneitaP noitacifitnedi 99 d. Transportation 100 e. Review of the chart 101 f. Surgical consent 102 g. refsnarT 104 h. Positioning 105 i. Urinary catheterization 106 j. Skin preparation 108 k. Equipment 110 l. Instrumentation 112 2. Sterile a. Asepsis and sterile technique 113 b. Hand hygiene and surgical scrub 115 c. Gowning and gloving 116 d. Surgical counts 117 e. Draping 118 B. Intraoperative: Sterile 1. Specimen care 119 2. Abdominal incisions 121 3. Hemostasis 122 4. Exposure 123 5. Catheters and drains 124 6. Wound closure 128 7. Surgical dressings 137 8. Wound healing 140 1 c. Light regulation d. Photoreceptors e. Macula lutea f. Fovea centralis g. Optic disc h. Brain pathways C. Ear 1. Anatomy a. External ear (1) Auricle (pinna) (2) Tragus b. Middle ear (1) Ossicles (a) Malleus (b) Incus (c) Stapes (2) Oval window (3) Round window (4) Mastoid sinus (5) Eustachian tube c. Internal ear (1) Labyrinth (2) Cochlea 2. Physiology of hearing a. Sound wave reception b. Bone conduction c. -

Surgical Treatments for Obstructive Sleep Apnea (OSA) Policy Number: PG0056 ADVANTAGE | ELITE | HMO Last Review: 06/01/2021
Surgical Treatments for Obstructive Sleep Apnea (OSA) Policy Number: PG0056 ADVANTAGE | ELITE | HMO Last Review: 06/01/2021 INDIVIDUAL MARKETPLACE | PROMEDICA MEDICARE PLAN | PPO GUIDELINES This policy does not certify benefits or authorization of benefits, which is designated by each individual policyholder terms, conditions, exclusions and limitations contract. It does not constitute a contract or guarantee regarding coverage or reimbursement/payment. Self-Insured group specific policy will supersede this general policy when group supplementary plan document or individual plan decision directs otherwise. Paramount applies coding edits to all medical claims through coding logic software to evaluate the accuracy and adherence to accepted national standards. This medical policy is solely for guiding medical necessity and explaining correct procedure reporting used to assist in making coverage decisions and administering benefits. SCOPE X Professional _ Facility DESCRIPTION Sleep apnea is a disorder where breathing nearly or completely stops for periods of time during sleep. In obstructive sleep apnea (OSA), the brain sends the message to breathe, but there is a blockage to air flowing into the chest. It is a condition in which repetitive episodes of upper airway obstruction occur during sleep. The obstruction may be localized to one or two areas, or may encompass the entire upper airway passages to include the nasal cavity (nose), oropharynx (palate, tonsils, tonsillar pillars) and hypopharynx (tongue base). The hallmark symptom of OSA is excessive daytime sleepiness, and the typical clinical sign of OSA is snoring, which can abruptly cease and be followed by gasping associated with a brief arousal from sleep. The snoring resumes when the patient falls back to sleep, and the cycle of snoring/apnea/arousal may be repeated as frequently as every minute throughout the night. -

Bleeding As the Main Complication After Adenoidectomy And
© Borgis New Med 2015; 19(4): 125-129 DOI: 10.5604/14270994.1191787 Bleeding as the main complication after adenoidectomy and adenotonsillotomy Teresa Ryczer, *Lidia Zawadzka-Głos, Paulina Czarnecka, Katarzyna Sobczyk Department of Pediatric Otolaryngology, Medical University of Warsaw, Poland Head of Department: Lidia Zawadzka-Głos, MD, PhD summary Introduction. adenoidectomy and adenotonsillotomy are one of the most common surgeries performed in children due to adenoid and tonsils hypertrophy. although the complications after the surgery are quite rare, one of the most common com- plication is bleeding. Aim. the aim of the study was to analyze the rate of bleeding as the most common early complication (within 24 hours) after adenoidectomy and adenotonsillotomy. the assessed factors were: age, sex, type of surgery, frequency of bleeding and applied surgical treatment, as well as coexisting coagulation disorders. Material and methods. the retrospective analysis of clinical data of 1312 patients hospitalized in the department of pediatric otolaryngology of medical University of Warsaw between January 2011 and december 2012 who underwent adenoidectomy or adenotonsillotomy was done. the objective of the study was to analyze the rate of bleeding as the most common early com- plication (within 24 hours) after adenoidectomy and adenotonsillotomy. the assessed factors were: age, sex, type of surgery, frequency of bleeding and applied surgical treatment, coexisting coagulation disorders. Results. intense bleeding (p < 0.01) and complications requiring surgical treatment (p < 0.05) occured more often after ad- enotonsillotomy than after adenotomy. in patients with coexisting coagulation disorders early complications were observed more often (p < 0.01). patients from specific age groups did not demonstrate statisticaly relevant higher complication rate, nor did male versus female group (p > 0.05). -

Coders' Desk Reference for ICD-10-PCS Procedures
2 0 2 DESK REFERENCE 1 ICD-10-PCS Procedures ICD-10-PCS for DeskCoders’ Reference Coders’ Desk Reference for ICD-10-PCS Procedures Clinical descriptions with answers to your toughest ICD-10-PCS coding questions Sample 2021 optum360coding.com Contents Illustrations ..................................................................................................................................... xi Introduction .....................................................................................................................................1 ICD-10-PCS Overview ...........................................................................................................................................................1 How to Use Coders’ Desk Reference for ICD-10-PCS Procedures ...................................................................................2 Format ......................................................................................................................................................................................3 ICD-10-PCS Official Guidelines for Coding and Reporting 2020 .........................................................7 Conventions ...........................................................................................................................................................................7 Medical and Surgical Section Guidelines (section 0) ....................................................................................................8 Obstetric Section Guidelines (section -

Revision Rates and Speech Outcomes Following Pharyngeal Flap Surgery for Velopharyngeal Insufficiency
Research Original Investigation Revision Rates and Speech Outcomes Following Pharyngeal Flap Surgery for Velopharyngeal Insufficiency Dhave Setabutr, MD; Christina T. Roth, MS, CCC-SLP; David D. Nolen, MD; Brian Cervenka, MD; Jonathan M. Sykes, MD; Craig W. Senders, MD; Travis T. Tollefson, MD, MPH IMPORTANCE Velopharyngeal insufficiency in children with cleft palate (and other causes) contributes to difficulty with communication and quality of life. The pharyngeal flap is a workhorse to address hypernasality and nasal air escape. However, there is a paucity of literature on the characteristics of cases that require revision. OBJECTIVE To measure the revision rate of pharyngeal flaps, compare the preperceptual and postperceptual speech scores, and identify the characteristics of those patients who required revision. DESIGN, SETTING, AND PARTICIPANTS A retrospective medical record review was completed for patients who underwent pharyngeal flap surgery from June 1, 2008, through January 31, 2013, at a tertiary academic center. MAIN OUTCOMES AND MEASURES Perceptual speech analyses and surgical revision rates. Perceptual speech patterns before and after surgery were compared using nasal air emission and resonance scores. The association between requiring revision surgery and covariates was analyzed using multivariable mixed-effects logistic regression. RESULTS Sixty-one patients were identified, including 24 boys (39%) and 37 girls (61%). The mean (SD) patient age at the time of pharyngeal flap surgery was 8.2 (6.8) years (range, 3-55 years). Velopharyngeal insufficiency was associated with cleft palate in 51 patients (84%), and 17 patients (28%) had a syndrome. The mean (SD) time to surgery after the speech evaluation was 225 (229) days (range, 14-1341 days). -

Pediatric Adenoidectomy
5/14/16 Pediatric Adenoidectomy: Clinical Update Shraddha Mukerji, MD, FACS Pediatric Otolaryngology Assistant Professor Baylor College of Medicine, Texas Children’s Hospital 05/14/2016 Talk Is Focused on • Clinical symptoms of large adenoids • Indications for adenoidectomy: updated guidelines • Complications and contra-indications 1 5/14/16 Basic Anatomy Relationship to Paranasal Sinuses and Eustachian Tube Paranasal sinus 2 5/14/16 Clinical Symptoms of Large Adenoids/Adenoid Inflammation Recurrent Sinusis or chronic Nasal sinusis symptoms ETD, AOM or Nasal obstruc;on OME Hyponasal speech Middle ear Mouth breathing problems Adenoid facies Adenoid Facies Long pinched nose Nasal obstruction Palatal and alveolar Crowding of teeth problems Mouth breathing 3 5/14/16 When to Perform Adenoidectomy in Children? • Nasal obstruction • Sleep disordered breathing, obstructive sleep apnea • Recurrent otitis media, otitis media with effusion • Recurrent or chronic sinusitis Case Scenario • A 2-year-old male comes for evaluation of symptoms of SDB: snoring, mouth breathing, restless sleeper • PE: normal weight child, 1+ tonsils, no turbinate hypertrophy 4 5/14/16 Next Step… • Intranasal steroid spray • Adenoid evaluation • Sleep study Adenoid Evaluation 5 5/14/16 Adenoidectomy is commonly performed for nasal obstruction and sleep apnea with or without tonsillectomy Adenoidectomy for OME and Recurrent AOM 6 5/14/16 Otitis Media and Adenoid Removal: Updated Guidelines Previous guidelines Newer guidelines • Adenoidectomy was performed for • If the child is LESS THAN 4 a child with otitis media who was YEARS, adenoidectomy is not undergoing a SECOND set of recommended even if the child is tubes, irrespective of age and nasal having a second set of tubes, symptoms. -

Icd-9-Cm (2010)
ICD-9-CM (2010) PROCEDURE CODE LONG DESCRIPTION SHORT DESCRIPTION 0001 Therapeutic ultrasound of vessels of head and neck Ther ult head & neck ves 0002 Therapeutic ultrasound of heart Ther ultrasound of heart 0003 Therapeutic ultrasound of peripheral vascular vessels Ther ult peripheral ves 0009 Other therapeutic ultrasound Other therapeutic ultsnd 0010 Implantation of chemotherapeutic agent Implant chemothera agent 0011 Infusion of drotrecogin alfa (activated) Infus drotrecogin alfa 0012 Administration of inhaled nitric oxide Adm inhal nitric oxide 0013 Injection or infusion of nesiritide Inject/infus nesiritide 0014 Injection or infusion of oxazolidinone class of antibiotics Injection oxazolidinone 0015 High-dose infusion interleukin-2 [IL-2] High-dose infusion IL-2 0016 Pressurized treatment of venous bypass graft [conduit] with pharmaceutical substance Pressurized treat graft 0017 Infusion of vasopressor agent Infusion of vasopressor 0018 Infusion of immunosuppressive antibody therapy Infus immunosup antibody 0019 Disruption of blood brain barrier via infusion [BBBD] BBBD via infusion 0021 Intravascular imaging of extracranial cerebral vessels IVUS extracran cereb ves 0022 Intravascular imaging of intrathoracic vessels IVUS intrathoracic ves 0023 Intravascular imaging of peripheral vessels IVUS peripheral vessels 0024 Intravascular imaging of coronary vessels IVUS coronary vessels 0025 Intravascular imaging of renal vessels IVUS renal vessels 0028 Intravascular imaging, other specified vessel(s) Intravascul imaging NEC 0029 Intravascular -

Obstructive Sleep Apnea and the Role of Tongue Reduction Surgery in Children with Beckwith-Wiedemann Syndrome (2018)
RESEARCH INSTITUTE Obstructive sleep apnea and the role of tongue reduction surgery in children with Beckwith-Wiedemann syndrome (2018) Christopher M. Cielo, Kelly A. Duffy, Aesha Vyas, Jesse A. Taylor, Jennifer M. Kalish Background Patients with Beckwith-Wiedemann syndrome (BWS) can be affected by a large tongue (macroglossia). Similar to other features of BWS, macroglossia can vary in severity between patients. Studies suggest that children with macroglossia are at an increased risk for obstructive sleep apnea (OSA), a condition that is also highly variable, ranging from mild sleep obstruction to severe respiratory distress. No recommendations regarding OSA management in patients with BWS and macroglossia exist. Purpose This article reviews all available evidence regarding children with Beckwith-Wiedemann Syndrome (BWS) and macroglossia. The prevalence of obstructive sleep apnea (OSA) and management strategies in this population are discussed. Findings Evaluations Children suspected of having BWS and macroglossia should receive the following evaluations. No clear guidelines exist for at what age children should be evaluated. • Clinical Genetics: Any child with a feature suggestive of BWS should be referred to a clinical geneticist, who can evaluate the patient and determine whether the patient meets criteria for a clinical diagnosis of BWS. • Plastic Surgery: Patients with macroglossia should be referred to a plastic surgeon, who can evaluate the size of the tongue to determine whether a tongue reduction surgery is necessary. • Pulmonology: A pulmonologist can evaluate the degree to which the large tongue affects breathing, as an increased tongue size can narrow the airway and cause upper airway obstruction. o Polysomnography (sleep study) is used for evaluation of OSA in children and has been used in certain studies of BWS children to detect the following: moderate- severe OSA, upper airway obstruction, apnea, upper airway resistance, severe desaturation, sleep-disordered breathing, and snoring. -

Outcomes of Tongue Base Reduction and Lingual Tonsillectomy for Residual Pediatric Obstructive Sleep Apnea After Adenotonsillectomy
Published online: 2019-05-28 THIEME Original Research e415 Outcomes of Tongue Base Reduction and Lingual Tonsillectomy for Residual Pediatric Obstructive Sleep Apnea after Adenotonsillectomy Seckin Ulualp1 1 Department of Otolaryngology, University of Texas Southwestern Address for correspondence Seckin Ulualp, MD, Department of Medical Center at Dallas, Dallas, Texas, United States Otolaryngology, University of Texas Southwestern Medical Center at Dallas, 5323 Harry Hines Blvd, Dallas, TX 75390, United States Int Arch Otorhinolaryngol 2019;23:e415–e421. (e-mail: [email protected]). Abstract Introduction Upper airway obstruction at multiple sites, including the velum, the oropharynx, the tongue base, the lingual tonsils, or the supraglottis, has been resulting in residual obstructive sleep apnea (OSA) after tonsillectomy and adenoidectomy (TA). The role of combined lingual tonsillectomy and tongue base volume reduction for treatment of OSA has not been studied in nonsyndromic children with residual OSA after TA. Objective To evaluate the outcomes of tongue base volume reduction and lingual tonsillectomy in children with residual OSA after TA. Methods A retrospective chart review was conducted to obtain information on history and physical examination, past medical history, findings of drug-induced sleep endoscopy (DISE), of polysomnography (PSG), and surgical management. Pre- and postoperative PSGs were evaluated to assess the resolution of OSA and to determine the improvement in the obstructive apnea-hypopnea index (oAHI) before and after the surgery. Results A total of 10 children (5 male, 5 female, age range: 10–17 years old, mean age: 14.5 Æ 2.6 years old) underwent tongue base reduction and lingual tonsillectomy. Drug-induced sleep endoscopy (DISE) revealed airway obstruction due to posterior displacement of the tongue and to the hypertrophy of the lingual tonsils.