SEP 27 1994 Science
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

07 Chapter2.Pdf
22 METHODOLOGY 2.1 INTRODUCTION TO ELECTROCHEMICAL TECHNIQUES Electrochemical techniques of analysis involve the measurement of voltage or current. Such methods are concerned with the interplay between solution/electrode interfaces. The methods involve the changes of current, potential and charge as a function of chemical reactions. One or more of the four parameters i.e. potential, current, charge and time can be measured in these techniques and by plotting the graphs of these different parameters in various ways, one can get the desired information. Sensitivity, short analysis time, wide range of temperature, simplicity, use of many solvents are some of the advantages of these methods over the others which makes them useful in kinetic and thermodynamic studies1-3. In general, three electrodes viz., working electrode, the reference electrode, and the counter or auxiliary electrode are used for the measurement in electrochemical techniques. Depending on the combinations of parameters and types of electrodes there are various electrochemical techniques. These include potentiometry, polarography, voltammetry, cyclic voltammetry, chronopotentiometry, linear sweep techniques, amperometry, pulsed techniques etc. These techniques are mainly classified into static and dynamic methods. Static methods are those in which no current passes through the electrode-solution interface and the concentration of analyte species remains constant as in potentiometry. In dynamic methods, a current flows across the electrode-solution interface and the concentration of species changes such as in voltammetry and coulometry4. 2.2 VOLTAMMETRY The field of voltammetry was developed from polarography, which was invented by the Czechoslovakian Chemist Jaroslav Heyrovsky in the early 1920s5. Voltammetry is an electrochemical technique of analysis which includes the measurement of current as a function of applied potential under the conditions that promote polarization of working electrode6. -

Hydrodynamic Voltammetry As a Rapid and Simple Method for Evaluating Soil Enzyme Activities
Sensors 2015, 15, 5331-5343; doi:10.3390/s150305331 OPEN ACCESS sensors ISSN 1424-8220 www.mdpi.com/journal/sensors Article Hydrodynamic Voltammetry as a Rapid and Simple Method for Evaluating Soil Enzyme Activities Kazuto Sazawa 1,* and Hideki Kuramitz 2 1 Center for Far Eastern Studies, University of Toyama, Gofuku 3190, 930-8555 Toyama, Japan 2 Department of Environmental Biology and Chemistry, Graduate School of Science and Engineering for Research, University of Toyama, Gofuku 3190, 930-8555 Toyama, Japan; E-Mail: [email protected] * Author to whom correspondence should be addressed; E-Mail: [email protected]; Tel./Fax: +81-76-445-66-69. Academic Editor: Ki-Hyun Kim Received: 26 December 2014 / Accepted: 28 February 2015 / Published: 4 March 2015 Abstract: Soil enzymes play essential roles in catalyzing reactions necessary for nutrient cycling in the biosphere. They are also sensitive indicators of ecosystem stress, therefore their evaluation is very important in assessing soil health and quality. The standard soil enzyme assay method based on spectroscopic detection is a complicated operation that requires the removal of soil particles. The purpose of this study was to develop a new soil enzyme assay based on hydrodynamic electrochemical detection using a rotating disk electrode in a microliter droplet. The activities of enzymes were determined by measuring the electrochemical oxidation of p-aminophenol (PAP), following the enzymatic conversion of substrate-conjugated PAP. The calibration curves of β-galactosidase (β-gal), β-glucosidase (β-glu) and acid phosphatase (AcP) showed good linear correlation after being spiked in soils using chronoamperometry. -

Linear Sweep Voltammetric Determination of Free Chlorine in Waters Using Graphite Working Electrodes
J.Natn.Sci.FoundationLinear sweep voltammetric Sri Lanka determination 2008 36 (1):of free 25-31 chlorine 25 RESEARCH ARTICLE Linear sweep voltammetric determination of free chlorine in waters using graphite working electrodes K.A.S. Pathiratne*, S.S. Skandaraja and E.M.C.M. Jayasena Department of Chemistry, Faculty of Science, University of Kelaniya, Kelaniya. Revised: 16 June 2007 ; Accepted: 23 July 2007 Abstract: Applicability of linear sweep voltammetry using and stored. During disinfecting processes, it is added graphite working electrodes for determination of free chlorine in to potable waters and it undergoes hydrolysis in water waters was demonstrated. Influence of the nature of supporting forming hypochlorous acid and hypochlorous ions. electrolyte, its concentrations, pH and rate of potential variation of working electrode on voltammetric responses corresponding - NaOCl + H O HOCl + NaOH (1) to the oxidation of ClO were examined. It was found that, any 2 of the salt solutions KNO , K SO or Na SO at the optimum 3 2 4 2 4 Kd concentration of 0.1 mol dm-3 could be used as a supporting HOCl ClO- + H+ (2) electrolyte for the above determination. The study also revealed (Dissociation Constant, K = 2.9 x 10-8 mol dm-3) that, any pH in the range of 8.5 to 11 could yield satisfactory d results. The anodic peak current at the working electrode potential of +1.030 V vs Ag/AgCl reference electrode was found As shown by equation (2), hypochlorous acid to linearly increase with concentration of free chlorine up to undergoes further dissociation and depending on the pH, 300 mg dm-3 (R2 = 0.9996). -

Linear Sweep Anodic Stripping Voltammetry: Determination of Chromium (VI) Using Synthesized Gold Nanoparticles Modified Screen-Printed Electrode
J. Chem. Sci. Vol. 127, No. 6, June 2015, pp. 1075–1081. c Indian Academy of Sciences. DOI 10.1007/s12039-015-0864-4 Linear sweep anodic stripping voltammetry: Determination of Chromium (VI) using synthesized gold nanoparticles modified screen-printed electrode SALAMATU ALIYU TUKURa,b, NOR AZAH YUSOFa,c,∗ and REZA HAJIANc,∗ aDepartment of Chemistry, Faculty of Science, Universiti Putra Malaysia, 43400 UPM, Serdang, Selangor, Malaysia bDepartment of Chemistry, Faculty of Science, Kaduna State University, Kaduna, Nigeria cInstitute of Advanced Technology, Universiti Putra Malaysia, Serdang, Selangor 43400, Malaysia e-mail: [email protected]; [email protected] MS received 16 October 2014; revised 17 February 2015; accepted 19 February 2015 Abstract. A highly sensitive electrochemical sensor has been constructed for determination of Cr(VI) with the lowest limit of detection (LOD) reported to date using gold nanoparticles (AuNPs) modified screen-printed electrode (SPE). The modification of SPE by casting pure AuNPs increases the sensitivity for detection of Cr(VI) ion using anodic stripping voltammetry. Cr(VI) ions are reduced to chromium metal on SPE-AuNPs by applying deposition potential of –1.1 V for 180 s. Afterwards, the oxidation peak current of chromium is obtained by linear sweep voltammetry in the range of −1.0 V to 0.2 V. Under the optimized conditions (HClO4, 0.06 mol L−1; deposition potential, –1.1 V; deposition time, 180s; scan rate, 0.1 V s−1), the limit of detection (LOD) was 1.6 pg mL−1. The fabricated electrode was successfully used for detection of Cr(VI) in tap and seawater. -

Cyclic Voltammetry
Cyclic Voltammetry Denis Andrienko January 22, 2008 2 Literature: 1. Allen J. Bard, Larry R. Faulkner “Electrochemical Methods: Fundamentals and Applications” 2. http://www.cheng.cam.ac.uk/research/groups/electrochem/teaching.html Chapter 1 Cyclic Voltammetry 1.1 Background Cyclic voltammetry is the most widely used technique for acquiring qualitative information about elec- trochemical reactions. it offers a rapid location of redox potentials of the electroactive species. A few concepts has to be introduced before talking about this method. 1.1.1 Electronegativity Electronegativity is the affinity for electrons. The atoms of the various elements differ in their affinity for electrons. The term was first proposed by Linus Pauling in 1932 as a development of valence bond theory. The table for all elements can be looked up on Wikipedia: http://en.wikipedia.org/wiki/Electronegativity. Some facts to remember: • Fluorine (F) is the most electronegative element. χF = 3.98. • The electronegativity of oxygen (O) χO = 3.44 is exploited by life, via shuttling of electrons between carbon (C, χF = 2.55) and oxygen (O): Moving electrons against the gradient (O to C) - as occurs in photosynthesis - requires energy (and stores it). Moving electrons down the gradient (C to O) - as occurs in cellular respiration - releases energy. • The relative electronegativity of two interacting atoms plays a major part in determining what kind of chemical bond forms between them. Examples: • Sodium (χNa = 0.93) and Chlorine (χCl = 3.16) = Ionic Bond: There is a large difference in electronegativity, so the chlorine atom takes an electron from the sodium atom converting the atoms into ions (Na+) and (Cl−). -
Waveneuro 4 Fast-Scan Cyclic Voltammetry (FSCV) Potentiostat System
WaveNeuro 4 Fast-Scan Cyclic Voltammetry (FSCV) Potentiostat System Pine Research Instrumentation 2741 Campus Walk Avenue Building 100 Durham, NC 27705 USA http://www.pineresearch.com Phone: +1 919.782.8320 Fax: +1 919.782.8323 Copyright © 2008 - 2019 Pine Research Instrumentation DRU10227 (REV001 / FEB 2019) Table of Contents 1. PREFACE 1 1.1 Scope .................................................................................................................................... 1 1.2 Copyright .............................................................................................................................. 1 1.3 Trademarks ........................................................................................................................... 1 1.4 Use Limitation ....................................................................................................................... 1 1.5 Service and Warranty Information .................................................................................... 2 1.6 Icons (Icônes) ....................................................................................................................... 3 1.7 Back Panel Markings ........................................................................................................... 4 1.7.1 Serial Number ............................................................................................................................. 4 1.7.2 Model Numbers ......................................................................................................................... -

Mica and Mineral Insulated Band Heaters
Mica and Mineral Insulated Band Heaters Mica and Mineral Insulated Band Heaters Mica & Mineral Insulated Band Heaters from National Plastic Heater are available in various sizes, voltages, wattage's, and constructions to suit every application. The Mica Insulated Band Heater is used for temperatures to 900ºF. The Mineral Insulated Band Heaters is used for temperatures to 1400ºF. The Mica and Mineral Band Heater can be internally or externally heated, full or split case for easy removal or installed and supplied with various types of leads as shown below. Mica & Mineral Insulated Band Heater Features: Sheath temperatures to 1400ºF (760ºC) mineral insulated • Split case design for easy removal and installation • Nichrome ribbon resistance wire precision wound • Mica/mineral insulation thin construction for quick heat transfer • Contamination resistant closed ended heater construction • Superior heat transfer due to minimum spacing between wire and sheath • Single set of leads possible on split case heaters Mica & Mineral Band Heater Specifications Design Capabilities: Dimensions. Minimum Inner Diameter 0.750" Maximum Diameter Consult NPH Maximum width 2 X diameter Consult NPH Voltage. 12 volts to 600 Volts AC/DC 1 or 3 Phase Watt Densities. Maximum to 100 watts/in Mineral Insulated Maximum Current. .30 Amps Post Terminals 8.5 Amps per Pair Options. Holes along the heater at required location Cutouts or slots of various dimensions Partial Coverage for sectional heating Larger Gaps at clamping end for sensors etc. Ground Wire or Lug for safety 2 Piece Split Case or Hinged construction European Plug / Terminal Box Internal Thermocouple. Available J, K Thermocouple Location. .Sheath To Order a Mica & Mineral Band Heater Please Provide: Watts, Volts, Inner Diameter, Width, Lead Length, Type of leads, Options. -

Historical Introduction Summarized History of Air Heating and Sheathed Heating Elements
Jacques Jumeau English version History of technologies linked to heating. Chapter 5 Historical introduction Summarized history of air heating and sheathed heating elements Copyright: Jacques Jumeau 1st edition 2015/11/26 Copy is allowed, provided you cite the origin: Ultimheat Museum 1 Update 2019/02/20 Historical introduction Summarized history of air heating and sheathed heating elements The invention of sheathed heating elements comprising a metal tube swaged around a coiled heating wire, and which is insulated by compressed magnesia, was an essential step of the electrothermics development. Thanks to their mechanical strength, impermeability and resistance to corrosion, these are the most professional heating technical solutions. The appearance of these heating elements, now universally used, was the result of a combination of different advanced techniques of the early 20th Century. Over the last two decades of the 19th Century, the emergence of electric heating had revealed the need to find reliable solutions for converting electricity into heat. The first electrical heaters were platinum wires (inherited laboratory equipment), nickel silver or even iron. Research carried on resistive elements with greater resistivity and good temperature resistance. On October 12, 1878, St. George Lane Fox-Pitt filed patent in England 4043, in which he developed the use of electricity for lighting and heating. This patent, based on the use of platinum filaments, was not followed for heating but it was the basis for the development of electric bulbs. In 1884, French Henri Marbeau, a pioneer in the manufacture of Nickel in New Caledonia and France, founded the company "Le Ferro-Nickel'' in Lizy sur Ourcq". -

For Peer Review Only
IUPAC Terminology of electrochemical methods of analysis Journal: Pure and Applied Chemistry ManuscriptFor ID PeerPAC-REC-18-01-09.R2 Review Only Manuscript Type: Recommendation Date Submitted by the 27-Feb-2019 Author: Complete List of Authors: Pingarron, José; Complutense University of Madrid, Department of Analytical Chemistry Labuda, Jan; Slovak University of Technology, Department of Analytical Chemistry Barek, Jiří ; Charles University, Faculty of Science, Department of Analytical Chemistry Brett, Christopher; Universidade de Coimbra, Departamento de Química Camões, Maria; Universidade de Lisboa, Centro de Química Estrutural Fojta, Miroslav; Akademie ved Ceské Republiky, Institute of Biophysics Hibbert, David; University of New South Wales, School of Chemistry electoanalytical chemistry, electrodes, potentiometry, amperometry, Keywords: voltammetry, coulometry, electrogravimetry, conductometry, impedimetry, spectroelectrochemistry Author-Supplied Keywords: P.O. 13757, Research Triangle Park, NC (919) 485-8700 Page 1 of 62 IUPAC 1 2 3 4 IUPAC Provisional Recommendation 5 6 7 José M. Pingarrón1, Ján Labuda2, Jiří Barek3, Christopher M.A. Brett4, Maria Filomena 8 Camões5, Miroslav Fojta6, D. Brynn Hibbert7*. 9 10 11 Terminology of electrochemical methods of analysis (IUPAC 12 Recommendations 201x) 13 14 15 16 Abstract: Recommendations are given concerning the terminology of methods used in 17 electroanalytical chemistry.For PeerFundamental Review terms in electrochemistry Only are reproduced from 18 previous PAC Recommendations, and -

Resistance Heating Alloy Nichrome 80 ( UNS N06003)
Resistance Heating Alloy Nichrome 80 ( UNS N06003) Durable Nichrome 80 resistance alloy is a traditional heating material for the industrial applications. It heats up quickly upon the passage of electricity. High service temperature up to 1200oC. Great oxidation and corrosion resistance at the elevated temperatures. It is commonly used in heating wires, coils, hair dryers, ovens, atomizers, metal sheath tubular elements and precision heating applications. Nichrome Ni80Cr20 has lower high resistance per foot. It offers more increment in resistance per foot with increasing temperature. Chemical Composition Nickel (Ni) 80 % Chromium (Cr) 20 % Physical Properties Density 8.31 g/cm3 or 0.303 lb/in3 Electrical resistivity 108 microhm • cm at 20oC 650 ohm. Circ. Mil/ft at 20oC Highest service temperature 1200oC or 2190oF Melting temperature 1400oC or 2550oF Coefficient of thermal expansion 12.5 micro-m per m oC from 20oC to 100oC Nichrome 80 Electric resistivity data AWG size Diameter (inch) Resistance (ohms/ft) lb / 1000 ft Ft per lb 8 0.129 inch 0.039 ohms/ft 47.23 lb / 1000 ft 22 Ft per lb 9 0.114 inch 0.050 ohms/ft 37.44 lb / 1000 ft 26 Ft per lb 10 0.102 inch 0.063 ohms/ft 29.71 lb / 1000 ft 33 Ft per lb 11 0.091 inch 0.080 ohms/ft 23.53 lb / 1000 ft 44 Ft per lb 12 0.081 inch 0.099 ohms/ft 18.72 lb / 1000 ft 55 Ft per lb 13 0.072 inch 0.125 ohms/ft 14.83 lb / 1000 ft 66 Ft per lb 14 0.064 inch 0.160 ohms/ft 11.75 lb / 1000 ft 86 Ft per lb 15 0.057 inch 0.200 ohms/ft 9.33 lb / 1000 ft 108 Ft per lb 16 0.051 inch 0.252 ohms/ft 7.4 lb / 1000 ft 135 Ft per lb 17 0.045 inch 0.317 ohms/ft 5.9 lb / 1000 ft 171 Ft per lb 18 0.040 inch 0.400 ohms/ft 4.65 lb / 1000 ft 214 Ft per lb 19 0.036 inch 0.504 ohms/ft 3.69 lb / 1000 ft 271 Ft per lb Heanjia Super-Metals Co., Ltd, Call- 12068907337. -
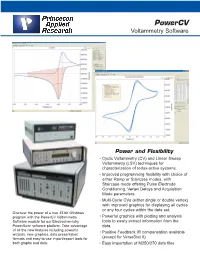
Powercv Voltammetry Software
PowerCV Voltammetry Software Power and Flexibility • Cyclic Voltammetry (CV) and Linear Sweep Voltammetry (LSV) techniques for characterization of redox-active systems. • Improved programming flexibility with choice of either Ramp or Staircase modes, with Staircase mode offering Pulse Electrode Conditioning, Vertex Delays and Acquisition Mode parameters. • Multi-Cycle CVs (either single or double vertex) with improved graphics for displaying all cycles or any four cycles within the data set. Discover the power of a true 32-bit Windows program with the PowerCV Voltammetry • Powerful graphics with plotting and analysis Software module for our Electrochemistry tools to easily extract information from the PowerSuite software platform. Take advantage data. of all the new features including powerful • Positive Feedback IR compensation available wizards, new graphics, data presentation formats and easy-to-use import/export tools for (except for VersaStat II) both graphs and data. • Easy importation of M250/270 data files Discover the Power in PowerCV 32 PowerCV continues our long tradition of providing the leading voltammetry software to the research electrochemistry community. PowerCV, the PowerSuite module that offers Cyclic Voltammetry (CV) and Linear Sweep Volatmmetry (LSV) techniques for data acquisition and analysis with Princeton Applied Research systems, has expanded its capabilities further with the addition of new waveform programming options and analysis tools. LSV and CV are the most widely used techniques in electrochemical studies as they provide a fast and simple way to characterize a redox-active system. In these techniques, the potentiostat applies a linear sweep of a programmed potential range and monitors the current as a function of the applied potential. -

Electrochemical Methods of Analysis Thomas Wenzel Department of Chemistry Bates College, Lewiston ME 04240 [email protected]
Electrochemical Methods of Analysis Thomas Wenzel Department of Chemistry Bates College, Lewiston ME 04240 [email protected] The following textual material is designed to accompany a series of in-class problem sets that develop many of the fundamental aspects of electrochemical analytical methods. TABLE OF CONTENTS 1. Basic Concepts in Electrochemistry 2 2. The Chemical Energy of a System 4 3. Relationship of Chemical Energy to Electrochemical Potential 10 4. Table of Standard State Electrochemical Potentials 12 5. Electrochemical Cells 14 6. Potential of an Electrochemical Cell 20 7. Electrochemical Analytical Methods 24 7.1. Ion-Selective Electrodes 25 7.1.1. pH Electrode 25 7.1.2. Other Glass Electrodes 27 7.1.3. Membrane Electrodes 27 7.1.4. Enzyme Electrodes 28 7.1.5. Solid-State Electrodes 28 7.1.6. Gas-Sensing Electrodes 28 7.2. Electrodeposition/Electrogravimetry 29 7.3. Coulometry 31 7.4. Titrimetric Methods of Analysis 33 7.4.1. “Classical” Redox Titration 33 7.4.2. Coulometric Titration (Controlled Current Coulometry) 33 7.4.3. Amperometric Titration 35 7.4.4. Potentiometric Titration 37 7.5. Voltammetric Methods 44 7.5.1. Anodic Stripping Voltammetry 46 7.5.2. Linear Sweep Voltammetry 49 7.5.3. Differential Pulse Linear Sweep Voltammetry 52 7.5.4. Cyclic Voltammetry 55 1 1. Basic Concepts in Electrochemistry Electrochemical processes are commonly used for analytical measurements. There are a variety of electrochemical methods with different degrees of utility for quantitative and qualitative analysis that are included in this unit. The coverage herein is not exhaustive and methods that are most important or demonstrate different aspects of electrochemical measurements are included.