Response Mechanism of Oryza Sativa to Boron and Nacl Stresses
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Presidents of Frontal Organizations and Cells
Presidents of Frontal Organizations and Cells 1. Shri.Shafi Parambil MLA 9446091115 President, Youth Congress A-19, New Civil Nagar, Palakkad 2. Shri.Abhijith.K.M 9447030202 President, KSU Vadakkedath House, Eramangalam PO Kozhikode-673612 3 Smt.Lathika Subhash 0481-2310990/ President, Mahila Congress 0484-2488277 Lakshmi Nivas, Kumaranalloor P.O. 9446534990 Kottayam 4. Chief Organiser Seva Dal 5 Shri. R.Chandrasekharan 0476 2851155 President INTUC 9447157455 Ayikkomathu House Sooranad North Sooranad P.O. Kollam. 6. Dr.SS Lal +12023049926 President All India Professional's Congress 7. Adv.Savin Sathyan 9744811234 Chairman All India Unorganized Workers Congress Ushas, Ezhukone PO, Kollam-691505 8. Shri.N.Venugopal 0484 2221800 Chairman 0484 2226360 Rajiv Gandhi Panchayat Raj Sangathan 94470 26830 Srilakam, Pandarachira, 1 st Cross Road, Kadavanthara PO, Kochi-20 9. Shri.G.V Hari 0472 2800222 Chairman, Jawahar Bala Jana Vedi 94472 43811 Neeharam, TC.50/447, F-12 98950 06811 Karamana PO, Thiruvananthapuram 10 Chairman, Kerala Pradesh Minority Cell 11 Shri.B.S.Shiju 9871598812 Director, RGIDS Shiji Manzil, Ambalammukku Kummil PO, Kadakkal, Kollam-691536 12 Shri.Aryadan Shoukath 9747000070 Chairman, Samskara Sahithi 9946000600 Aryadan House, Nilambur P.O, 04931 220324 Malappuram 13 Adv.K.K.Manoj 04868-264422 President, Adivassi Congress 9447178822 Karinchayil House, Thoprankudy P.O, Idukki 14 Shri.Karakulam Krishna Pillai 9447413366 Chairman, Sahakarana Janathipatiya Vedi Prasanth, Karakulam PO, Trivandrum 15 Adv. T.Asafali 9447757614 President, Indian Lawyers Congress Emai:[email protected] C-1/407, Mareena Majestic Goshri Bridge Road, Cochin-682018 16 Shri. Nedumudi Harikumar 9447303976 Convener, Vichar Vibhag Priyadarsini, Palace Ward, Alappuzha 11 17 Lt.Col.S.R.Bhuvanendran Nair, 9995755255 Chairman Ex. -

Accused Persons Arrested in Alappuzha District from 18.10.2020To24.10.2020
Accused Persons arrested in Alappuzha district from 18.10.2020to24.10.2020 Name of Name of the Name of the Place at Date & Arresting Court at Sl. Name of the Age & Cr. No & Sec Police father of Address of Accused which Time of Officer, which No. Accused Sex of Law Station Accused Arrested Arrest Rank & accused Designation produced 1 2 3 4 5 6 7 8 9 10 11 Rajeev Bhavanam, Cr No- 1060 / 24-10-2020 BAILED BY 1 Narayanan NairRamakrishnan Nair63 Male Venmony thazham Thazhathambalam 2020 U/s 118 VENMANI Pradeep S 22:30 POLICE Muri, Venmony village (a) of KP Act Cr No- 835 / Pulatharayil,Potahapp 24-10-2020 2020 U/s 279 KB BAILED BY 2 viswanathan opalan 60 Male ally THRIKKUNNAPPUZHA THRIKUNNAPUZHA 19:29 ipc & 185 of ANANDABABU POLICE sout,Kumarapuram Mv act GEETHA BHAVAN, Cr No- 980 / PAZHAVEEDU P O, 24-10-2020 BIJU K R, S I OF BAILED BY 3 NANDAKUMARPADMAKUMAR18 Male NEAR IRON BRIDGE 2020 U/s 279 ALAPPUZHA SOUTH THIRUVAMBADY 19:22 POLICE POLICE IPC WARD, ALAPPUZHA Cr No- 1474 / 2020 U/s 188, 269 IPC & 118(e) of KP Act & Sec. KL MAHESH, SI Nedumpallil,Peringala, 24-10-2020 BAILED BY 4 Riyas Muhammed Kutty44 Male STORE JUNCTION 4(2)(a) r/w 5 of MANNAR OF POLICE , kayamkulam 18:50 POLICE Kerala MANNNAR Epidemic Diseases Ordinance 2020 Cr No- 1473 / 2020 U/s 188, 269 IPC & 118(e) of KP Act & Sec. KL MAHESH, SI padinjar Kuttiyil 24-10-2020 BAILED BY 5 Akhil raj Ashok Kumar 23 Male STORE JUNCTION 4(2)(a) r/w 5 of MANNAR OF POLICE , Gramam,Ennakkadu 18:40 POLICE Kerala MANNNAR Epidemic Diseases Ordinance 2020 Chandralayam, Cr No- 979 / 24-10-2020 BIJU -
Cluster Training 2020
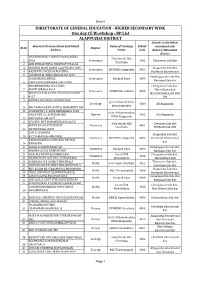
Sheet1 DIRECTORATE OF GENERAL EDUCATION - HIGHER SECONDARY WING One day CE Workshop - RP List ALAPPUZHA DISTRICT Schools in the below Resource Persons Name and School Name of Training School mentioned sub Sl No Subject Address Centre Code district/ Education district DILEEPKUMAR V SNHSS POOCHAKKAL Holy family HSS 1 4046 Economics 4061 Thuravoor Sub dist Cherthala 2 ANILKUMAR VJHSS NADUVATH NAGAR 3 MANJU K MANI GGHSS ALAPPUZHA 4095 Alappuzha Sub dist, Economics SDVBHSS alappuzha 4052 4 RASIYATH LMHSS ALAPPUZHA Kuttanad Educational 5 SATHYAN M GHSS MANGALAM 4019 Ambalappuzha Sub dist, SUJIKUMARI SNTHSS Economics Haripad Boys 4004 Harippad Sub dist 6 NANGIARKULANGARA ,PALLIPAD KRISHNAKUMAR K K GGHSS Chengannur Sub dist , 7 MAVELIKKARA 4013 Mavelikkara Sub Economics GBHSS Mavelikkara 4093 ANITHA G PILLAI NSS HSS KURATHIKAD dist,KAYAMKULAM SUB 8 4127 DIS 9 AMBIKA BAI,GHSS CHANDIROOR gov.mohammedans Sociology 4030 All Alappuzha ghss,alappuzha 10 BIJI DAMODARAN SNTHSS MARARIKULAM 11 VISWAJITH P S GHSS KIDANGARA 4026 Govt. Muhammedans AMAR EBY A L SAMAJAM HSS History 4012 All Alappuzha BHSS Alappuzha 12 MUTHUKULAM 4077 13 JAYAN.N SNT MARARIKULAM (4072) Holy family HSS Cherthala Sub dist BOBIN K PALIATH SFAHSS Chemistry 4061 Cherthala THURAVOOR SUB 14 ARTHUNKAL(4047) SAM R SNDPHSS Alappuzha Sub dist, 15 KUTTAMANGALAM(4035) Chemistry SDVBHSS alappuzha 4052 Kuttanad Educational RADHAKRISHNA PANICKER NSS HSS dist 16 KAVALAM 17 SANAL R GGHSS HARIPAD Ambalappuzha Sub dist, Chemistry Haripad Boys 4004 18 ANJANA GGHSS KAKKAZHAM Harippad Sub dist 19 RANI.M.S -

Accused Persons Arrested in Alappuzha District from 13.12.2020To19.12.2020
Accused Persons arrested in Alappuzha district from 13.12.2020to19.12.2020 Name of Name of the Name of the Place at Date & Arresting Court at Sl. Name of the Age & Cr. No & Sec Police father of Address of Accused which Time of Officer, which No. Accused Sex of Law Station Accused Arrested Arrest Rank & accused Designation produced 1 2 3 4 5 6 7 8 9 10 11 Cr.1010/2020 U/s Sec. 4(2)(j) r/w 5 of Kerala 19 NIKATHIL VEEDU, 19-12-2020 KAREELAKUL SUNUMON K, JFMC II 1 RENJU RAVEENDRAN CHINGOLI Epidemic Male CHINGOLI 21:05 ANGARA SI OF POLICE HARIPAD Diseases Ordinance 2020 CHAKKANATT THARA MUTTATHIPPARAMBU ASHOK RAMANKUTTY 52 VETANARY JN , 19-12-2020 Cr.2683/2020 JFMC I 2 P O CHERTALA THOMAS T.J KUMAR VYDYAR Male CHERTHALA 20:50 U/s 279 IPC CHERTHALA THANNEERMUKKOM P/W-17 manappally colony thekkekkara Cr.1344/2020 48 19-12-2020 Rajeev , si of JFMC II 3 ANILKUMAR Thankan kizhakkumuri Pallipad U/s 118(a) of HARIPPAD Male 17:25 police HARIPAD Pallippad p/w-3 KP Act pallippad village Cr.2682/2020 PULITHARAKAZHNARA PURUSHOTHA 25 19-12-2020 U/s 279 IPC & C.B BABU, SI OF JFMC I 4 VISHNU M PATTANAKKAD P O OLATHALA CHERTALA MAN Male 14:25 128,129 OF POLICE CHERTHALA VAYALAR P/W- MV ACT Cr.2680/2020 U/s 269 IPC & 118(e) of KP VELIYIL HOUSE Act & Sec. MANUSANKA 23 THIRUNALLOOR P O THIRUNALLOO 19-12-2020 4(2)(j) r/w 5 of ASOKAN C R, SI JFMC I 5 CHANDRADAS CHERTALA R Male CHENNAM R 12:00 Kerala OF POLICE CHERTHALA PALLIPPURAM P/W-10 Epidemic Diseases Ordinance 2020 Cr.2679/2020 U/s 269 IPC & 118(e) of KP Act & Sec. -
Kuttanad Report.Pdf
Measures to Mitigate Agrarian Distress in Alappuzha and Kuttanad Wetland Ecosystem A Study Report by M. S. SWAMINATHAN RESEARCH FOUNDATION 2007 M. S. SWAMINATHAN RESEARCH FOUNDATION FOREWORD Every calamity presents opportunities for progress provided we learn appropriate lessons from the calamity and apply effective remedies to prevent its recurrence. The Alappuzha district along with Kuttanad region has been chosen by the Ministry of Agriculture, Government of India for special consideration in view of the prevailing agrarian distress. In spite of its natural wealth, the district has a high proportion of population living in poverty. The M. S. Swaminathan Research Foundation was invited by the Union Ministry of Agriculture to go into the economic and ecological problems of the Alappuzha district as well as the Kuttanad Wetland Ecosystem as a whole. The present report is the result of the study undertaken in response to the request of the Union Ministry of Agriculture. The study team was headed by Dr. S. Bala Ravi, Advisor of MSSRF with Drs. Sudha Nair, Anil Kumar and Ms. Deepa Varma as members. The Team was supported by a panel of eminent technical advisors. Recognising that the process of preparation of such reports is as important as the product, the MSSRF team held wide ranging consultations with all concerned with the economy, ecological security and livelihood security of Kuttanad wetlands. Information on the consultations held and visits made are given in the report. The report contains a malady-remedy analysis of the problems and potential solutions. The greatest challenge in dealing with multidimensional problems in our country is our inability to generate the necessary synergy and convergence among the numerous government, non-government, civil society and other agencies involved in the implementation of the programmes such as those outlined in this report. -

List of Notified Areas(Panchayats/Muni./Corp) Notified for Paddy ( Autumn ) Kharif 2020,2021 & 2022 Seasons
Annexure PM‐K‐I List of Notified Areas(Panchayats/Muni./Corp) Notified for Paddy ( Autumn ) Kharif 2020,2021 & 2022 Seasons Notified SL No District Block Notified Panchayat List of Villages Crops 1 AMBALAPUZHA AMBALAPUZHA (N) Paddy All Villages in the Notified Panchayat 2 ALAPPUZHA MUNI. ,PUNNAPRA (N) Paddy All Villages in the Notified Panchayats 3 PURAKKAD Paddy All Villages in the Notified Panchayat 4 AMBALAPUZHA (S) Paddy All Villages in the Notified Panchayat 5 PUNNAPRA (S) Paddy All Villages in the Notified Panchayat 6 ARYAD ARYAD ,MANNANCHERY Paddy All Villages in the Notified Panchayats 7 MUHAMMA Paddy All Villages in the Notified Panchayat 8 MARARIKULAM (S) Paddy All Villages in the Notified Panchayat 9 BHARANIKKAVU MAVELIKARA (MUNI.) Paddy All Villages in the Notified Panchayat 10 KANJIKUZHY CHERTHALA Paddy All Villages in the Notified Panchayat 11 CHERTHALA (S) Paddy All Villages in the Notified Panchayat 12 KANJIKUZHI Paddy All Villages in the Notified Panchayat 13 THANNEERMUKKOM Paddy All Villages in the Notified Panchayat 14 KADAKKARAPPALLY Paddy All Villages in the Notified Panchayat 15 MARARIKULAM (N) Paddy All Villages in the Notified Panchayat 16 PATTANAKKAD AROOR Paddy All Villages in the Notified Panchayat 17 KODAMTHURUTH Paddy All Villages in the Notified Panchayat 18 PATTANAKKAD Paddy All Villages in the Notified Panchayat 19 EZHUPUNNA Paddy All Villages in the Notified Panchayat 20 KUTHIYATHODE Paddy All Villages in the Notified Panchayat 21 THURAVOOR Paddy All Villages in the Notified Panchayat 22 VAYALAR Paddy -

Survival of Xanthomonas Oryzae Pv. Oryzae (Ishiyama
Journal of Tropical Agriculture 39 (2001): 76-78 SURVIVAL OF XANTHOMONAS ORYZAE PV. ORYZAE (ISHIYAMA, 1922), SWINGS ETAL., 1990 Bacterial blight caused by Xanthomonas variety at maximum tillering stage by using an oryzae pv. oryzae (Ishiyama, 1922), Swings et aqueous suspension containing chopped stub- al., 1990 is one of the most important diseases bles. The clip inoculation was done periodi- of rice in South East Asia. In Kerala, this dis- cally at seven days interval for a total period ease is prevalent in two of the major rice of 63 days. Three replications were main- growing tracts viz., Palakkad and Kuttanad. tained for each location. Observations were In Kuttanad, this disease occurs in a mild form made on the occurrence of typical symptoms during punja season (November - December to of bacterial blight such as yellowing and mar- February - March) and in a severe form during ginal blighting of leaves after seven days of the additional crop season (June - July to Sep- inoculation. The final data were recorded as tember - October). The present investigation positive or negative for the development of was taken up to study the mode of survival of symptoms of bacterial blight. the pathogen in infected seed and stubbles and their role in the recurrence of bacterial blight In addition to this, infected stubbles under disease in Kuttanad mainly during the addi- field condition were studied. For this, bacte- tional crop season. rial blight infected stubbles were maintained under natural field conditions both under dry Infected seeds were collected from paddy and wet land conditions at four different loca- fields of Champakulam, Edathua, Kainakary, tions viz., Champakulam, Nedumudi, Pulin- Kavalam, Muttar, Nedumudi, Pulinkunnu, kunnu and Ramankari. -

Alappuzha District, Kerala State
TECHNICAL REPORTS: SERIES ‘D’ CONSERVE WATER – SAVE LIFE भारत सरकार GOVERNMENT OF INDIA जल संसाधन मंत्रालय MINISTRY OF WATER RESOURCES कᴂ द्रीय भूजल बो셍 ड CENTRAL GROUND WATER BOARD केरल क्षेत्र KERALA REGION भूजल सूचना पुस्ततका, आलपषाु स्ज쥍ला, केरल रा煍य GROUND WATER INFORMATION BOOKLET OF ALAPPUZHA DISTRICT, KERALA STATE तत셁वनंतपरु म Thiruvananthapuram December 2013 GOVERNMENT OF INDIA MINISTRY OF WATER RESOURCES CENTRAL GROUND WATER BOARD GROUND WATER INFORMATION BOOKLET OF ALAPPUZHA DISTRICT, KERALA 饍वारा By सररता एस Saritha S. Asst. Hydrogeologist सहायक भूजलवि嵍य KERALA REGION BHUJAL BHAVAN KEDARAM, KESAVADASAPURAM NH-IV, FARIDABAD THIRUVANANTHAPURAM – 695 004 HARYANA- 121 001 TEL: 0471-2442175 TEL: 0129-12419075 FAX: 0471-2442191 FAX: 0129-2142524 GROUND WATER INFORMATION BOOKLET OF ALAPPUZHA DISTRICT, KERALA STATE TABLE OF CONTENTS DISTRICT AT A GLANCE 1.0 INTRODUCTION ....................................................................................................... 1 2.0 CLIMATE AND RAINFALL..................................................................................... 3 3.0 GEOMORPHOLOGY AND SOIL TYPES................................................................ 5 4.0 GROUNDWATER SCENARIO ................................................................................ 6 5.0 GROUNDWATER MANAGEMENT STRATEGY ................................................ 14 6.0 GROUNDWATER RELATED ISSUES AND PROBLEMS.................................. 15 7.0 AWARENESS AND TRAINING ACTIVITY ....................................................... -

Accused Persons Arrested in Alappuzha District from 06.09.2020To12.09.2020
Accused Persons arrested in Alappuzha district from 06.09.2020to12.09.2020 Name of Name of the Name of the Place at Date & Arresting Court at Sl. Name of the Age & Cr. No & Sec Police father of Address of Accused which Time of Officer, which No. Accused Sex of Law Station Accused Arrested Arrest Rank & accused Designation produced 1 2 3 4 5 6 7 8 9 10 11 Cr No- 1437 Valantharanikarth /2020 U/s 40 12-09-2020 BAILED BY 1 Riyas Abdulkhadar Arookutty P O Poochakkal 188,269 IPC POOCHAKAL Sudarshan C K Male 20:30 POLICE Arookutty P W II 4(2)(j) r/w 5 of KEDO 2020 VALIYA VILAYIL Cr No- 181 SUMESH SASIDARAN 39 KANJIRATHODU THIRUVAMPAD 12-09-2020 /2020 U/s 279 TRAFFIC PS BAILED BY 2 SI MOHANDAS KUMAR PILLA Male VALLIKUNNAM P/W Y 19:20 IPC & 185 MV ALAPPUZHA POLICE VII ACT Cr No- 1122 /2020 U/s 143,147,149,1 GOKULAM 88,269,270 VIJAYAN 34 HOUSE,UMBERNADU 12-09-2020 MAVELIKKAR Prasad K.K, SI BAILED BY 3 ABHILASH MAVELIKKARA ipc,118(e) OF PILLAI Male MURI,THAZHAKKARA 19:00 A of Police POLICE KP VILLEGE ACT,4(2)(e)(j) r/w 5 of KEDO 2020 Cr No- 1122 /2020 U/s MELETHU 143,147,149,1 KIZHAKKATHIL,VAAHU 88,269,270 MAHESH MOHANAN 34 12-09-2020 MAVELIKKAR Prasad K.K, SI BAILED BY 4 VADI MAVELIKKARA ipc,118(e) OF MOHANAN PILLAI Male 19:05 A of Police POLICE MURI,THAZHAKKARA KP VILLEGE ACT,4(2)(e)(j) r/w 5 of KEDO 2020 Cr No- 1122 /2020 U/s AJEESH 143,147,149,1 BHAVANAM,KARAZHA 88,269,270 51 12-09-2020 MAVELIKKAR Prasad K.K, SI BAILED BY 5 SHAJI KUTTAPPAN MA MAVELIKKARA ipc,118(e) OF Male 19:15 A of Police POLICE MURI,VALLIKUNNAM KP VILLEGE ACT,4(2)(e)(j) r/w -

Accused Persons Arrested in Alappuzha District from 04.10.2020To10.10.2020
Accused Persons arrested in Alappuzha district from 04.10.2020to10.10.2020 Name of Name of Name of the Place at Date & Arresting the Court Sl. Name of the Age & Cr. No & Police father of Address of Accused which Time of Officer, at which No. Accused Sex Sec of Law Station Accused Arrested Arrest Rank & accused Designation produced 1 2 3 4 5 6 7 8 9 10 11 Cr No - 852 / 2020 U/s 188, 269,270 IPC & Sec. 4(2)(J) ALAKKOTTU,MUTTAM 10-10-2020 r/w 5 of KAREELAKUL SIVAPRASAD,SI BAILED BY 1 SHIBU BINOY Male 40 CHUNDUPALAKA JN P.O 19:00 Kerala ANGARA OF POLICE POLICE Epidemic Diseases Ordinance 2020 Cr No - 852 / 2020 U/s 188, 269,270 IPC & Sec. 4(2)(J) PLAKKATTU 10-10-2020 r/w 5 of KAREELAKUL SIVAPRASAD,SI BAILED BY 2 GIREESHKUMARLEKSHMANAN Male 44 CHUNDUPALAKA JN HOUSE,MUTTAM P.O 19:00 Kerala ANGARA OF POLICE POLICE Epidemic Diseases Ordinance 2020 Cr No - 852 / 2020 U/s 188, 269,270 IPC & Sec. 4(2)(J) PLAKKATTU 10-10-2020 r/w 5 of KAREELAKUL SIVAPRASAD,SI BAILED BY 3 GOPALAKRISHNANRAGHAVAN Male 57 PADEETATHIL,MUTTA CHUNDUPALAKA JN 19:00 Kerala ANGARA OF POLICE POLICE M P.O Epidemic Diseases Ordinance 2020 Cr No - 852 / 2020 U/s 188, 269,270 IPC & Sec. 4(2)(J) CHITHIRA,MUTTAM 10-10-2020 r/w 5 of KAREELAKUL SIVAPRASAD,SI BAILED BY 4 SHIBULAL PRABHAKARANMale 41 CHUNDUPALAKA JN P.O 19:00 Kerala ANGARA OF POLICE POLICE Epidemic Diseases Ordinance 2020 Cr No - 852 / 2020 U/s 188, 269,270 IPC & Sec. -

Rehabilitation & Resettlement
UPDATED RESETTLEMENT ACTION PLAN Public Disclosure Authorized KERALA STATE HIGHWAY PROJECT- II PUBLIC WORKS DEPARTMENT Public Disclosure Authorized GOVERNMENTOFKERALA DECEMBER 2012 Public Disclosure Authorized Public Disclosure Authorized EXCUTIVE SUMMARY 1. PROJECT PURPOSE The Kerala State Transport Project (KSTP II) aims to improve the performance of the State's road transport network by upgrading the road conditions and capacity, together with development of the in house capabilities of the Kerala State Public Works Department (PWD) to plan, develop and maintain the road network. The KSTP I was designed to upgrade 581 km and cover 1000 km under maintenance in two Phases. However, due to several reasons including delay in land acquisition, only 254 km of Phase I road was upgraded and maintenance works for 1180 km were carried out from June 2002 and December 2009. Even though civil works on remaining 327 km for upgradation of Phase II roads could not be undertaken, land acquisition and Resettlement and Rehabilitation (R&R) of affected people continued. As a result, the preparation phase of KSTP II coincides with the implementation of Resettlement Action Plan for Phase II roads (see Addendum I) which was approved by Government of Kerala and World Bank in 2003. Therefore, the Government of Kerala decided to prepare "Updated RAP" which comprises of the following: 1. Executive Summary 2. Addendum I - RAP Phase II 3. Addendum II - Progress report on implementation of RAP and action plan for balance activities 4. Annexures 2. Project Location KSTP II constitutes link numbers 68, 69, 74,4,5,84.1 and 84.2 which were selected in 2003 and link 41 & 47 was included in 2009 (see table below). -
List of Supplyc Outlets with Phone No
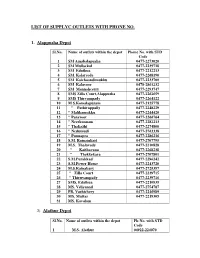
LIST OF SUPPLYC OUTLETS WITH PHONE NO. 1. Alappuzha Depot Sl.No. Name of outlets within the depot Phone No. with STD Code 1 SM Amabalapuzha 0477-2273020 2 SM Mullackal 0477-2239718 3 SM Edathua 0477-2212213 4 SM. Kalarcode 0477-2268190 5 SM Kaichoondimukku 0477-2233700 6 SM Kalavoor 0478-2861232 7 SM Mannahcerry 0477-2293747 8 SMS Zilla Court,Alappuzha 0477-2262659 9 SMS Thirvampady 0477-2264122 10 M S.Komalapuram 0477-2125778 11 " Pathirappally 0477-2248229 12 " Malikamukku 0477-2244420 13 " Paravoor 0477-2266764 14 " Neerkunnam 0477-2282213 15 " Thakazhi 0477-2274800 16 " Nedumudi 0477-2763338 17 " Punnapra 0477-2286216 18 S.M. Ramamkari 0477-2707795 19 M.S. Thalavady 0477-2210828 20 " Kaithavana 0477-2268238 21 " Thekkekara 0477-2707801 22 S.M.Purakkad 0477-2296242 23 S.M.Power House 0477-2243720 24 M.S.Kainakary 0477-2725397 25 " Zilla Court 0477-2239715 26 " Thiruvampady 0477-2239716 27 SMS, Edathua 0477-2210935 28 MS, Veliyanad 0477-2754787 29 PB, Vazhichery 0477-2246989 30 MS, Muttar 0477-2219305 31 MS, Kavalam 2) Alathur Depot Sl.No. Name of outlets within the depot Ph:No. with STD Code 1 M.S. Alathur 04922-224070 2 " Karapotta 04922-267234 3 " Kavassery 04922-206736 4 " Kottayi 04922-285155 5 " Kuthanur 04922-240524 6 " Kunissery 04922-200754 7 " Mathur 04922-241236 8 " Vandazhy 04922-261366 9 " Melarcode (Kadampidi) 04922-200459 10 " Nainankkad (Kizhakkencherry) 04922-203783 11 " Pazhamabalacode 04922-206580 12 " Perungottukurissi 04922-217325 13 SM, Puthucode 04922-267233 14 SM Kuzhalmannam 04922-273480 15 SMS Vadakkenchhery 04922-203750 3) Attingal Depot Sl.No.