Life History Patterns of Three Estuarine Brittlestars (Ophiuroidea) at Cedar Key, Florida
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Jacksonville, Florida 1998 Odmds Benthic Community Assessment
JACKSONVILLE, FLORIDA 1998 ODMDS BENTHIC COMMUNITY ASSESSMENT Submitted to U.S. Environmental Protection Agency, Region 4 61 Forsyth St. Atlanta, Georgia 30303 Prepared by Barry A. Vittor & Associates, Inc. 8060 Cottage Hill Rd. Mobile, Alabama 36695 (334) 633-6100 November 1999 TABLE OF CONTENTS LIST OF TABLES ………………………………………….……………………………3 LIST OF FIGURES ……………………..………………………………………………..4 1.0 INTRODUCTION ………..…………………………………………………………..5 2.0 METHODS ………..…………………………………………………………………..5 2.1 Sample Collection And Handling ………………………………………………5 2.2 Macroinfaunal Sample Analysis ……………………………………………….6 3.0 DATA ANALYSIS METHODS ……..………………………………………………6 3.1 Assemblage Analyses ..…………………………………………………………6 3.2 Faunal Similarities ……………………………………………………….…….8 4.0 HABITAT CHARACTERISTICS ……………………………………………….…8 5.0 BENTHIC COMMUNITY CHARACTERIZATION ……………………………..9 5.1 Faunal Composition, Abundance, And Community Structure …………………9 5.2 Numerical Classification Analysis …………………………………………….10 5.3 Taxa Assemblages …………………………………………………………….11 6.0 1995 vs 1998 COMPARISONS ……………………………………………………..11 7.0 SUMMARY ………………………………………………………………………….13 8.0 LITERATURE CITED ……………………………………………………………..16 2 LIST OF TABLES Table 1. Station locations for the Jacksonville, Florida ODMDS, June 1998. Table 2. Sediment data for the Jacksonville, Florida ODMDS, June 1998. Table 3. Summary of abundance of major taxonomic groups for the Jacksonville, Florida ODMDS, June 1998. Table 4. Abundance and distribution of major taxonomic groups at each station for the Jacksonville, Florida ODMDS, June 1998. Table 5. Abundance and distribution of taxa for the Jacksonville, Florida ODMDS, June 1998. Table 6. Percent abundance of dominant taxa (> 5% of the total assemblage) for the Jacksonville, Florida ODMDS, June 1998. Table 7. Summary of assemblage parameters for the Jacksonville, Florida ODMDS stations, June 1998. Table 8. Analysis of variance table for density differences between stations for the Jacksonville, Florida ODMDS stations, June 1998. -

Tube Epifaum of the Polychaete Phyllopchaetopterus Socialis
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Repository Open Access to Scientific Information from Embrapa Estuarine, Coastal and Shelf Science (1995) 41, 91–100 Tube epifauna of the Polychaete Phyllochaetopterus socialis Claparède Rosebel Cunha Nalessoa, Luíz Francisco L. Duarteb, Ivo Pierozzi Jrc and Eloisa Fiorim Enumod aDepartamento de Zoologia, CCB, Universidade Federal de Pernambuco, 50670-901, Recife, PE, Brazil, bDepartamento de Zoologia, Instituto Biologia, C.P. 6109, Universidade Estadual de Campinas, 13.081-970, Campinas, SP, Brazil, cEmbrapa, NMA, Av. Dr. Julio Soares de Arruda, 803 CEP 13.085, Campinas, SP, Brazil and dProtebras, Rua Turmalina, 79 CEP 13.088, Campinas, SP, Brazil Received 8 October 1992 and in revised form 22 June 1994 Keywords: Polychaeta; tubes; faunal association; epifauna; São Sebastião Channel; Brazil Animals greater than 1 mm, found among tangled tubes of Phyllochaetopterus socialis (Chaetopteridae) from Araçá Beach, São Sebastião district, Brazil, were studied for 1 year, with four samples in each of four seasons. They comprised 10 338 individuals in 1722·7 g dry weight of polychaete tubes, with Echino- dermata, Polychaeta (not identified to species) and Crustacea as the dominant taxa. The Shannon–Wiener diversity index did not vary seasonally, only two species (a holothurian and a pycnogonid) showing seasonal variation. Ophiactis savignyi was the dominant species, providing 45·5% of individuals. Three other ophiuroids, the holothurian Synaptula hidriformis, the crustaceans Leptochelia savignyi, Megalobrachium soriatum and Synalpheus fritzmuelleri, the sipunculan Themiste alutacea and the bivalve Hiatella arctica were all abundant, but most of the 68 species recorded occurred sparsely. -

Key to the Common Shallow-Water Brittle Stars (Echinodermata: Ophiuroidea) of the Gulf of Mexico and Caribbean Sea
See discussions, stats, and author profiles for this publication at: https://www.researchgate.net/publication/228496999 Key to the common shallow-water brittle stars (Echinodermata: Ophiuroidea) of the Gulf of Mexico and Caribbean Sea Article · January 2007 CITATIONS READS 10 702 1 author: Christopher Pomory University of West Florida 34 PUBLICATIONS 303 CITATIONS SEE PROFILE All content following this page was uploaded by Christopher Pomory on 21 May 2014. The user has requested enhancement of the downloaded file. All in-text references underlined in blue are added to the original document and are linked to publications on ResearchGate, letting you access and read them immediately. 1 Key to the common shallow-water brittle stars (Echinodermata: Ophiuroidea) of the Gulf of Mexico and Caribbean Sea CHRISTOPHER M. POMORY 2007 Department of Biology, University of West Florida, 11000 University Parkway, Pensacola, FL 32514, USA. [email protected] ABSTRACT A key is given for 85 species of ophiuroids from the Gulf of Mexico and Caribbean Sea covering a depth range from the intertidal down to 30 m. Figures highlighting important anatomical features associated with couplets in the key are provided. 2 INTRODUCTION The Caribbean region is one of the major coral reef zoogeographic provinces and a region of intensive human use of marine resources for tourism and fisheries (Aide and Grau, 2004). With the world-wide decline of coral reefs, and deterioration of shallow-water marine habitats in general, ecological and biodiversity studies have become more important than ever before (Bellwood et al., 2004). Ecological and biodiversity studies require identification of collected specimens, often by biologists not specializing in taxonomy, and therefore identification guides easily accessible to a diversity of biologists are necessary. -

Two New Brittle Star Species of the Genus Ophiothrix
Caribbean Journal of Science, Vol. 41, No. 3, 583-599, 2005 Copyright 2005 College of Arts and Sciences University of Puerto Rico, Mayagu¨ez Two New Brittle Star Species of the Genus Ophiothrix (Echinodermata: Ophiuroidea: Ophiotrichidae) from Coral Reefs in the Southern Caribbean Sea, with Notes on Their Biology GORDON HENDLER Natural History Museum of Los Angeles County, 900 Exposition Boulevard, Los Angeles, California 90007, U.S.A. [email protected] ABSTRACT.—Two new species, Ophiothrix stri and Ophiothrix cimar, inhabit shallow reef-platforms and slopes in the Southern Caribbean, and occur together at localities in Costa Rica and Panama, nearly to Colombia. What appears to be an undescribed species resembling O. cimar has been reported from eastern Venezuela. In recent years, reefs where the species were previously observed have deteriorated because of environmental degradation. As a consequence, populations of the new species may have been reduced or eradicated. The new species have previously been mistaken for O. angulata, O. brachyactis, and O. lineata. Ophiothrix lineata, O. stri, and O. cimar have in common a suite of morphological features pointing to their systematic affinity, and a similar pigmentation pattern consisting of a thin, dark, medial arm stripe flanked by two pale stripes. Ophiothrix lineata is similar to Indo-Pacific members of the subgenus Placophiothrix and closely resembles Ophiothrix stri. The latter is extremely similar to O. synoecina, from Colombia, and both can live in association with the rock-boring echinoid Echinometra lucunter. Although O. synoecina is a protandric hermaphrodite that reportedly broods its young externally, the new species are gonochoric and do not brood. -

Echinoderm Species of Flower Garden Banks National Marine Sanctuary
CORAL CAP SPECIES OF FLOWER GARDEN BANKS NATIONAL MARINE SANCTUARY Classification Common name Scientific Name Echinoderms Brittle Stars Amphiodia pulchella Amphioplus tumidus Asteroschema sp. Astrocyclus caecilia Basket Star Astrophyton muricatum Asteroporpa annulata Gorgonocephalus sp. Ophiactis algicola Five-armed Ophiactis Ophiactis quinqueradia Savigny's Brittle Star Ophiactis savignyi Blunt-spined/Spiny Brittle Star Ophiocoma echinata Red Ophiocoma Ophiocoma wendtii Ophiocomella ophiactoides Banded-arm/Harlequin Brittle Star Ophioderma appressum Serpent Star Ophioderma brevispinum Ruby Brittle Star Ophioderma rubicundum Scaly Brittle Star, Red Serpent Star Ophioderma squamosissimum Elegant Brittle Star Ophiolepis elegans ?Ophiophragmus sp. Reticulated Brittle Star Ophionereis reticulata Ophiostigma isacanthus Angular Brittle Star Ophiothrix angulata Suenson's/Sponge Brittle Star Ophiothrix suensonii Ophiurochaeta littoralis Sea Cucumbers Five-toothed Sea Cucumber Actinopyga agassizii Beaded Sea Cucumber Euapta lappa Holothuria lentiginosa Chocolate Chip/Three-rowed Cucumber Isostichopus (Stichopus) badionotus Cucumber Thyone pseudofusus Sea Stars Astropecten comptus Asterinopsis lymani Asterinopsis pilosis Coronaster briareus Goniaster tessellatus Common Comet Star Linckia guildingii Linckia nodosa Guilding's/Comet Star Ophidiaster guildingii Sclerasterias contorta CORAL CAP SPECIES OF FLOWER GARDEN BANKS NATIONAL MARINE SANCTUARY Classification Common name Scientific Name Sea Urchins Purple-spined Sea Urchin Arbacia punctulata Centrostephanus longispinus rubricingulus Coelopleurus floridanus Long-spined Sea Urchin Diadema antillarum Slate-pencil Urchin Eucidaris tribuloides Genocidaris maculata Lytechinus euerces Green/Variegated Urchin Lytechinus variegatus Red Heart Urchin Meoma ventricosa Paraster doederleini Stained Collector Urchin Pseudoboletia maculata maculata Red Sea Urchin Stylocidaris affinis . -

Living Resources Report Texas A&M University-Corpus Christi Results - Open Bay Habitat
Center for Coastal Studies CCBNEP Living Resources Report Texas A&M University-Corpus Christi Results - Open Bay Habitat B. Living Resources - Habitats Detailed community profiles of estuarine habitats within the CCBNEP study area are not available. Therefore, in the following sections, the organisms, community structure, and ecosystem processes and functions of the major estuarine habitats (Open Bay, Oyster Reef, Hard Substrate, Seagrass Meadow, Coastal Marsh, Tidal Flat, Barrier Island, and Gulf Beach) within the CCBNEP study area are presented. The following major subjects will be addressed for each habitat: (1) Physical setting and processes; (2) Producers and Decomposers; (3) Consumers; (4) Community structure and zonation; and (5) Ecosystem processes. HABITAT 1: OPEN BAY Table Of Contents Page 1.1. Physical Setting & Processes ............................................................................ 45 1.1.1 Distribution within Project Area ......................................................... 45 1.1.2 Historical Development ....................................................................... 45 1.1.3 Physiography ...................................................................................... 45 1.1.4 Geology and Soils ................................................................................ 46 1.1.5 Hydrology and Chemistry ................................................................... 47 1.1.5.1 Tides .................................................................................... 47 1.1.5.2 Freshwater -
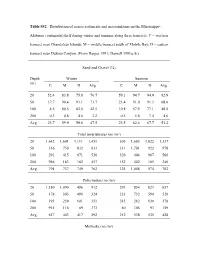
Table S32. Distribution of Coarse Sediments and Macroinfauna on the Mississippi
Table S32. Distribution of coarse sediments and macroinfauna on the Mississippi- Alabama continental shelf during winter and summer along three transects. C = western transect near Chandeleur Islands; M = middle transect south of Mobile Bay; D = eastern transect near DeSoto Canyon. (From Harper 1991; Darnell 1991a, b.) Sand and Gravel (%) Depth Winter Summer (m) C M D Avg. C M D Avg. 20 52.4 83.8 75.8 70.7 59.1 94.7 94.9 82.9 50 37.7 90.4 93.1 73.7 23.4 91.0 91.3 68.6 100 4.5 60.5 62.6 42.5 10.8 57.9 77.1 48.6 200 0.3 4.8 4.6 3.2 0.5 5.8 7.4 4.6 Avg. 23.7 59.9 59.0 47.5 23.5 62.4 67.7 51.2 Total invertebrates (no./m²) 20 1,642 1,601 1,111 1,451 505 1,683 1,822 1,337 50 316 750 832 633 311 1,701 922 978 100 291 415 871 526 326 404 967 566 200 946 183 182 457 152 402 185 246 Avg. 794 737 749 762 324 1,048 974 782 Polychaetes (no./m²) 20 1,180 1,090 486 912 293 854 823 657 50 178 305 490 324 233 732 594 520 100 193 259 601 351 243 262 630 378 200 915 116 89 373 80 305 93 159 Avg. 617 443 417 492 212 538 535 428 Mollusks (no./m²) 20 123 127 174 141 89 527 407 341 50 46 214 115 125 11 653 73 246 100 32 80 23 45 20 63 38 40 200 14 7 10 10 26 27 9 21 Avg. -

Echinoderm Research and Diversity in Latin America
Echinoderm Research and Diversity in Latin America Bearbeitet von Juan José Alvarado, Francisco Alonso Solis-Marin 1. Auflage 2012. Buch. XVII, 658 S. Hardcover ISBN 978 3 642 20050 2 Format (B x L): 15,5 x 23,5 cm Gewicht: 1239 g Weitere Fachgebiete > Chemie, Biowissenschaften, Agrarwissenschaften > Biowissenschaften allgemein > Ökologie Zu Inhaltsverzeichnis schnell und portofrei erhältlich bei Die Online-Fachbuchhandlung beck-shop.de ist spezialisiert auf Fachbücher, insbesondere Recht, Steuern und Wirtschaft. Im Sortiment finden Sie alle Medien (Bücher, Zeitschriften, CDs, eBooks, etc.) aller Verlage. Ergänzt wird das Programm durch Services wie Neuerscheinungsdienst oder Zusammenstellungen von Büchern zu Sonderpreisen. Der Shop führt mehr als 8 Millionen Produkte. Chapter 2 The Echinoderms of Mexico: Biodiversity, Distribution and Current State of Knowledge Francisco A. Solís-Marín, Magali B. I. Honey-Escandón, M. D. Herrero-Perezrul, Francisco Benitez-Villalobos, Julia P. Díaz-Martínez, Blanca E. Buitrón-Sánchez, Julio S. Palleiro-Nayar and Alicia Durán-González F. A. Solís-Marín (&) Á M. B. I. Honey-Escandón Á A. Durán-González Laboratorio de Sistemática y Ecología de Equinodermos, Instituto de Ciencias del Mar y Limnología (ICML), Colección Nacional de Equinodermos ‘‘Ma. E. Caso Muñoz’’, Universidad Nacional Autónoma de México (UNAM), Apdo. Post. 70-305, 04510, México, D.F., México e-mail: [email protected] A. Durán-González e-mail: [email protected] M. B. I. Honey-Escandón Posgrado en Ciencias del Mar y Limnología, Instituto de Ciencias del Mar y Limnología (ICML), UNAM, Apdo. Post. 70-305, 04510, México, D.F., México e-mail: [email protected] M. D. Herrero-Perezrul Centro Interdisciplinario de Ciencias Marinas, Instituto Politécnico Nacional, Ave. -

GC .S6 N~G ·E;~~ :.~"
GC 1000 .S6 n~g ·e;~~ :.~" GC 1000 .D65 E34 1979 EFFECTS OF DREDGING AND UNCONFINED DISPOSAL OF DREDGED MATERIAL ON MACROBENTHIC COMMUNITIES IN SEWEE BAY, SOUTH CAROLINAl by Robert F. Van Dolah Dale R. Calder David M. Knott Magdalene S. Maclin Marine Resources Center South Carolina Wildlife and Marine Resources Department Charleston, South Carolina 29412 Technical Report No. 39 South Carolina Marine Resources Center April 1979 Proper~y of csc Library 1 Study funded under contract (#DACW-60-77-C-0013) for the U. S. Army Corps of Engineers, Charleston District. US Department of Commerce NOP.A co~1.::.; tal Services Center Library 2234 South Hobson Avenue Charleston, SC 29405-2413 TABLE OF CONTENTS Page LIST OF FIGURES •••••••..••••......•.••••••••.••.•.••..•.••••..•.••.••••..•••••.•....•••••....•••••••..... iii LIST OF TABLES........................................................ iii INTRODUCTION. • • • • • . • . • • • • • . • • • . • . • . • . • . • • . • . • . 1 DESCRIPTION OF STUDY AREA. 1 METHODS.................................................................................................. 1 Benthic Sampling.................................................................................... 1 Benthic Analytical Techniques....................................................................... 1 Water Chemistry................................................. • • • . • • . • . • 3 RESULTS AND DISCUSSION................................................................................... 3 Water Chemistry, ••••••••• ,, •••••• ,,,,,,, -

An Association Between Hesione Picta (Polychaeta: Hesionidae)
Zoological Studies 51(6): 762-767 (2012) An Association between Hesione picta (Polychaeta: Hesionidae) and Ophionereis reticulata (Ophiuroidea: Ophionereididae) from the Brazilian Coast José Eriberto De Assis*, Emerson de Azevedo Silva Bezerra, Rafael Justino de Brito, Anne Isabelley Gondim, and Martin Lindsey Christoffersen Laboratório e Coleção de Invertebrados Paulo Young, Departamento de Sistemática e Ecologia, CCEN, Universidade Federal da Paraíba, João Pessoa, Paraíba, Brazil (Accepted February 22, 2012) José Eriberto De Assis, Emerson de Azevedo Silva Bezerra, Rafael Justino de Brito, Anne Isabelley Gondim, and Martin Lindsey Christoffersen (2012) An association between Hesione picta (Polychaeta: Hesionidae) and Ophionereis reticulata (Ophiuroidea: Ophionereididae) from the Brazilian coast. Zoological Studies 51(6): 762-767. This paper is the 1st report on the association between Hesione picta and Ophionereis reticulata, based on specimens found in the southwestern Atlantic, State of Paraíba, Brazil. The associated partners were found under rocks during low tide. We studied 30 specimens of O. reticulata, which harbored 30 hesionids. Observations of animals in aquaria showed that the polychaete responded to the presence of the ophiuroid, slowly crawling until it touched the latter. After recognizing its partner, the polychaete remained in its habitual position on the aboral surface of the ophiuroid, as also observed in the natural environment. On the other hand, when the hesionid was placed with other ophiuroid species which coexist at the same locality, the animals withdrew from each other. http://zoolstud.sinica.edu.tw/Journals/51.6/762.pdf Key words: Hesionid, Ophionereidid, Intertidal fauna. Both the ecology and ecological roles of ophiuroids and starfishes were found on the polychaete associations have recently received disc, arms, and even the oral cavity of the hosts increasing attention (Britayev 1989, Britayev et al. -

Planktonic Dispersal of Juvenile Brittle Stars (Echinodermata: Ophiuroidea) on a Caribbean Reef
BULLETIN OF MARINE SCIENCE, 65(1): 283–288, 1999 CORAL REEF PAPER PLANKTONIC DISPERSAL OF JUVENILE BRITTLE STARS (ECHINODERMATA: OPHIUROIDEA) ON A CARIBBEAN REEF Gordon Hendler, Carole C. Baldwin, David G. Smith and Christine E. Thacker ABSTRACT Juveniles of four species of shallow water ophiuroids were captured in a plankton net tethered on the reef flat at Carrie Bow Cay, Belize: Ophiothrix orstedii, Ophiothrix angulata, Ophiocoma wendtii, and Ophiactis savignyi. These water-borne animals were similar in size to the smallest benthic conspecifics found on the reef, but considerably larger than newly metamorphosed postlarvae. Thus, this first report of planktonic dis- persal by juvenile coral reef ophiuroids suggests that they reenter the plankton and drift, or perhaps raft on algal fragments, after first having recruited to the benthos. The occur- rence of water-borne juveniles in species with planktotrophic and abbreviated larval de- velopment, and with clonal, asexual reproduction suggests that postlarval drifting may augment larval dispersal in some ophiuroid species and substitute for it in others. It has been suggested that rafting dispersal, by adult marine invertebrates clinging to buoyant objects, prevails in cool waters where drift algae are abundant and float for long distances before deteriorating (Highsmith, 1985; Johannesson, 1988; Parker and Tunnicliffe, 1994). Long-distance larval dispersal may prevail in tropical waters where larvae can survive and delay metamorphosis for extended periods (Pechenik, 1980; Scheltema, 1995; Palumbi et al., 1997; Lessios et al., 1998). However, the geographic patterns of rafting and larval dispersal are more complex than suggested by that simplis- tic temperate-tropical dichotomy. They are shaped by the behavior and physiology of larvae and postlarvae (Yamaguchi, 1977; Pechenik, 1990) and other factors such as direc- tions and speeds of oceanic currents (Palumbi et al., 1997; Lessios et al., 1998). -
Echinodermata, Ophiuroidea)
A peer-reviewed open-access journal ZooKeys 307: 45–96A (2013) taxonomic guide to the brittle-stars (Echinodermata, Ophiuroidea)... 45 doi: 10.3897/zookeys.307.4673 RESEARCH ARTICLE www.zookeys.org Launched to accelerate biodiversity research A taxonomic guide to the brittle-stars (Echinodermata, Ophiuroidea) from the State of Paraíba continental shelf, Northeastern Brazil Anne I. Gondim1, Carmen Alonso1, Thelma L. P. Dias2, Cynthia L. C. Manso3, Martin L. Christoffersen1 1 Universidade Federal da Paraíba, Programa de Pós-Graduação em Ciências Biológicas (Zoologia), Labo- ratório de Invertebrados Paulo Young (LIPY), João Pessoa, PB.CEP. 58059-900, Brasil 2 Universidade Esta- dual da Paraíba, Laboratório de Biologia Marinha (LabMar), Departamento de Biologia, Campus I, Rua Baraúnas, 351, Bairro Universitário, CEP 58429-500, Campina Grande, PB, Brasil 3 Universidade Federal de Sergipe, Departamento de Biociências, Laboratório de Invertebrados Marinhos (LABIMAR). Av. Vereador Olimpio Grande S/nº, 49.500-000, Itabaiana, SE, Brasil Corresponding author: Anne I. Gondim ([email protected]) Academic editor: Yves Samyn | Received 13 January 2013 | Accepted 16 May 2013 | Published 10 June 2013 Citation: Gondim AI, Alonso C, Dias TLP, Manso CLC, Christoffersen ML (2013) A taxonomic guide to the brittle- stars (Echinodermata, Ophiuroidea) from the State of Paraíba continental shelf, Northeastern Brazil. ZooKeys 307: 45– 96. doi: 10.3897/zookeys.307.4673 Abstract We provide the first annotated checklist of ophiuroids from the continental shelf of the State of Paraíba, northeastern Brazil. Identification keys and taxonomic diagnoses for 23 species, belonging to 14 genera and 8 families, are provided. The material is deposited in the Invertebrate Collection Paulo Young, at the Federal University of Paraíba.