Phi Zeta Research Day
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

2012 Phi Zeta Proceedings
The Honor Society of Veterinary Medicine Epsilon Chapter November 7, 2012 Research Emphasis Day AUBURN UNIVERSITY COLLEGE OF VETERINARY MEDICINE PHI ZETA EPSILON CHAPTER COLLEGE OF VETERINARY MEDICINE AUBURN UNIVERSITY welcomes you to our PHI ZETA RESEARCH DAY FORUM November 7, 2012 We want to thank all the presenters, their co-investigators and mentors for their participation in this annual event. We also want to thank all sponsors for their generous support without which this event would not be possible: Department of Anatomy, Physiology, and Pharmacology Department of Clinical Sciences Department of Pathobiology Office of the Assoc. Dean for Research and Graduate Studies Office of the Associate Dean of Academic Affairs Office of the Dean PROGRAM PHI ZETA RESEARCH EMPHASIS DAY November 7, 2012 – Overton-Goodwin Center Graduate Student Platform Presentations (OEW) OEW 248 OEW 251 8:30 Amy Back 8:45 Allison Bradbury 8:45 Michelle Aono 9:00 Erfan Chowdhury 9:00 Heather Davis 9:15 Elaine Norton 9:15 Elizabeth Barrett 9:30 Fernanda Cesar 9:30 Hui Huang 9:45 Break 9:45 Break 10:00 Anil Poudel 10:00 Valeria Albanese 10:15 India Napier 10:15 Kh. S. Rahman 10:30 Rucha Gurjar 10:30 Margaret Salter 10:45 Victoria McCurdy 10:45 Wes Campbell 11:00-1:00 Poster Presentations- Overton Education Wing (Poster Session Presenters are present 11:00 – 12:00) Veterinary Student Platform Presentation (OEW 248) 1:30 Jeremy Fleming 1:45 Amelia Nuwer 2:00 Hannah Findlay 2:15 Kelsie Theis 2:30 Nikki McAdams 1 PROGRAM Post-graduate/Faculty Platform Presentations (OEW 248) 2:45 Deepa Bedi 3:00 Heather Gray-Edwards 3:15 Merrilee Holland 3:30 Jeremy Foote 3:45 Snack Break – Joy Goodwin Cafeteria 4:00 Keynote Lecture – Overton Auditorium – Dr. -

Ramiro Isaza, DVM, MS, MPH, DACZM (Updated October 29, 2013)
Curriculum Vitae Ramiro Isaza, DVM, MS, MPH, DACZM (Updated October 29, 2013) PRESENT POSITION: Associate Professor of Zoological Medicine (Graduate Faculty Member) CONTACT INFORMATION: ACADEMIC ADDRESS Department of Small Animal Clinical Sciences College of Veterinary Medicine PO Box 100126 University of Florida Gainesville, Florida 32610-0126 TELEPHONE (352) 294-4443 FAX (352) 392-6125 E-MAIL [email protected] EDUCATION: MPH 2011 College of Public Health and Health Professions University of Florida Gainesville, Florida MS 1993 College of Veterinary Medicine University of Florida Gainesville, Florida DVM 1988 New York State College of Veterinary Medicine Cornell University Ithaca, New York BS 1983 Zoology, with High Honors University of Florida Gainesville, Florida AA/AS 1979 Zoo Keeper Training Program Santa Fe Community College Gainesville, Florida POST-GRADUATE TRAINING IN ZOOLOGICAL MEDICINE: Residency 1992 Wildlife and Zoological Medicine College of Veterinary Medicine University of Florida Gainesville, Florida Internship 1990 Zoological Medicine Riverbanks Zoological Park Columbia, SC Ramiro Isaza Page 2 BOARD CERTIFICATION: Diplomate (#40 / 127) 1996 American College of Zoological Medicine VETERINARY LICENSURES: Florida, California APPOINTMENTS AND PROFESSIONAL EXPERIENCE: 2007-present Associate Professor Zoological Medicine Service Department of Small Animal Clinical Sciences College of Veterinary Medicine, University of Florida Gainesville, Florida 2003-2007 Assistant Professor Zoological Medicine Service Department of Small Animal -

Student Handbook for Students in the Professional Veterinary Program
2021-22 University of Florida College of Veterinary Medicine Student Handbook For Students in the Professional Veterinary Program Student Handbook 2021-2022 UF reserves the right to implement new regulations and policies not currently included in this document. The university will make a reasonable attempt to inform students of changes in regulations or policies. The most updated version of the student handbook can be found at: http://education.vetmed.ufl.edu/dvm-curriculum/student-handbook/ Contents I. Introduction ........................................................................................................................... 6 Our Mission ......................................................................................................................................................................... 6 Calendar ............................................................................................................................................................................... 6 II Resources ................................................................................................................................ 7 Alumni Affairs ..................................................................................................................................................................... 7 Auditing a Course ................................................................................................................................................................ 7 Accessing Cornerstone from Home .................................................................................................................................... -

Ian Keith Hawkins Present Rank: Assistant
Curriculum Vitae Ian K. Hawkins 1. ACADEMIC HISTORY AND PROFESSIONAL EXPERIENCE: Name: Ian Keith Hawkins Present Rank: Assistant Professor Proportion of Time Assignment: 80% Service 20% Research Tenure Status: Not tenured Administrative Title: Assistant Professor and Veterinary Pathologist (TVDIL 2015-present). Graduate Faculty Status: Not a member Highest Degrees: Doctor of Veterinary Medicine University of Missouri May 2007 Bachelor of Science, Zoology and Entomology The Ohio State University June 2003 Specialty Training and Certification: Diplomate American College of Veterinary Pathologists September 2011 Anatomic Pathology Residency University of Florida July 2010 Professional Licensure: Georgia Veterinary Medical License, Faculty License, 2015 – present USDA Accreditation, Category II Veterinarian, 2015 – present Diplomate, American College of Veterinary Pathologists, 2011 – present Royal College of Veterinary Surgeons, 2011 – present Ohio Veterinary Medical License, 2007 – present Academic Positions: 2015-present: Assistant Professor and Veterinary Pathologist, University of Georgia, College of Veterinary Medicine, Tifton Veterinary Diagnostic and Investigational Laboratory. Professional Positions: 2011-2015: Veterinary Pathologist, Bridge Pathology Limited, Bristol, United Kingdom. 2011: Veterinarian, Lindquist Veterinary Center, Kirksville, Missouri. 2007-2010: Anatomic Pathology Resident, University of Florida, College of Veterinary Medicine, Department of Infectious Diseases and Pathology, Gainesville, Florida. Awards and Scholarships: -
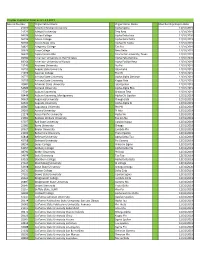
12.3.2019 LPH Chapter Expiration Dates.Xlsx
Chapter Expiration Dates as of 12.3.2019 Record Number Organization Name Organization Name Memberships Expire Date 21070 Abilene Christian University Alpha Sigma 12/31/2019 21523 Adelphi University Zeta Beta 12/31/2019 64336 Adrian College Alpha Delta Iota 12/31/2020 56196 Albion College Alpha Beta Delta 12/31/2019 66999 Alcorn State Univ Alpha Chi Alpha 12/31/2019 34827 Allegheny College Tau Eta 12/31/2019 20626 Alma College Beta Delta 12/31/2019 96670 Alpha Epsilon Rho Concordia University, Texas 12/31/2019 99780 American University in the Emirates Alpha Zeta Gamma 12/31/2020 69264 American University of Kuwait Alpha Epsilon Beta 12/31/2019 23243 Andrews University Nu Psi 12/31/2019 26793 Angelo State University Iota Alpha 12/31/2019 21079 Aquinas College Eta Chi 12/31/2019 26777 Arizona State University Alpha Alpha Omicron 12/31/2019 26799 Arizona State University Kappa Zeta 12/31/2019 20681 Arkansas State University Iota Upsilon 12/31/2019 54689 Ashland University Alpha Alpha Rho 12/31/2019 27741 Auburn University Omicron Zeta 12/31/2019 68446 Auburn University, Montgomery Alpha Chi Upsilon 12/31/2019 50629 Augsburg University Omega Zeta 12/31/2019 42631 Augusta University Alpha Alpha Xi 12/31/2019 40087 Augustana University Phi Phi 12/31/2019 29103 Aurora University Pi Iota 12/31/2019 21178 Azusa Pacific University Alpha Nu 12/31/2019 21061 Baldwin Wallace University Epsilon Nu 12/31/2019 37077 Ball State University Upsilon Kappa 12/31/2019 22981 Barry University Omega 12/31/2019 20627 Baylor University Lambda Phi 12/31/2019 21202 -

2-Pg. Curr. Vitae 11-3
CURRICULUM VITAE NANCY HUGHES ING May 11, 2015 Departments of Animal Science and Veterinary Integrative Biosciences Texas A&M University 2471 TAMU College Station, TX 77843-2471 Office Phone: (979) 862-2790 Home Telephone:(979) 690-6566 Fax: (979) 862-3399 e-mail: [email protected] EDUCATION: 1976-1979 B.S. University of Florida Zoology (with Honors) 1980-1984 D.V.M. University of Florida Veterinary Medicine 1980-1988 Ph.D. University of Florida Biochemistry & Molecular Biology 1988-1992 Post-Doc Baylor College of Medicine Cell Biology PROFESSIONAL APPOINTMENTS: 1986-1988 Research Assistant Department of Animal Science University of Missouri 1988-1992 Post-Doctoral Fellow Department of Cell Biology Baylor College of Medicine 1992- Assistant Professor Department of Animal Science Texas A&M University 1992- Joint Appointment Department of Veterinary Integrative Biosciences Texas A&M University 1998 Associate Professor Department of Animal Science Texas A&M University HONORS and AWARDS: 1975 Scholarship to attend the Summer Science Research Program of the Florida Foundation for Future Scientists at the University of Florida, American Cancer Society. 1979 Rita McTigue O'Connell Award Gainesville Women's Club 1979 Phi Beta Kappa University of Florida 1980 ERF Award American Medical Association 1980 Graduate Fellowship for Women Entering Non-Traditional Careers University of Florida 2000 Gamma Sigma Delta (Agricultural Honor Society) Texas A&M University 2004 Phi Beta Kappa (Founding Member) Texas A&M University 2005 Phi Zeta (Veterinary Honor -

Research Emphasis Day
The Honor Society of Veterinary Medicine Epsilon Chapter November 8, 2017 Research Emphasis Day AUBURN UNIVERSITY COLLEGE OF VETERINARY MEDICINE PHI ZETA EPSILON CHAPTER COLLEGE OF VETERINARY MEDICINE AUBURN UNIVERSITY Welcomes you to our PHI ZETA RESEARCH DAY FORUM November 8, 2017 We want to thank all the presenters, their co-investigators and mentors for their participation in this annual event. We also want to thank all sponsors for their generous support without which this event would not be possible: Office of the Dean Office of the Assoc. Dean for Research and Graduate Studies Auburn University Research Initiative in Cancer Department of Anatomy, Physiology, and Pharmacology Department of Clinical Sciences Department of Pathobiology Scott-Ritchey Research Center PHI ZETA RESEARCH DAY FORUM NOVEMBER 8, 2017 – VETERINARY EDUCATION CENTER 8:30 : Opening Statement To be determined 8:40-12:00 MORNING Presentations - Overton Auditorium Graduate Students and Residents – Moderator: to be determined 8:40 Abdul Mohin Sajib Evaluation of the Cancer-Specific Functionality of Canine Promoters to Expand Precision Medicine Approaches for Canine Tumors 8:52 Jack Kottwitz The Implications of Drugs Utilized for Assisted Breeding in Managed Rhinoceros 9:04 Randolph Winter Notched QRS complexes in dogs with and without structural cardiac disease: 85 cases 9:16 Annie Maguire Image Analysis of Sub-Gross Stains for a Feline Neurodegenerative Disease 9:28 Humberto Nobre Ante-mortem and Post-mortem diagnosis of Ovarian Follicular Dysplasia in Florida Beef -

Phi Zeta Epsilon Chapter College of Veterinary Medicine Auburn University
PHI ZETA EPSILON CHAPTER COLLEGE OF VETERINARY MEDICINE AUBURN UNIVERSITY welcomes you to our PHI ZETA RESEARCH DAY FORUM November 10, 2010 We want to thank all the presenters, their co-investigators and mentors for their participation in this annual event. We also want to thank all sponsors for their generous support without which this event would not be possible: VWR Daniel & Jessica Heard (on behalf of Qiagen) Fisher Scientific Department of Anatomy, Physiology, and Pharmacology Department of Clinical Sciences Department of Pathobiology Office of the Assoc. Dean for Research and Graduate Studies Office of the Dean PROGRAM PHI ZETA RESEARCH DAY FORUM NOVEMBER 10, 2010 - JOY GOODWIN RUDD STUDENT CENTER 8:30 : BREAKFAST Buffet - Goodwin Center Lobby 9-11: MORNING Presentations - Overton Auditorium Veterinary Students 9:00 B.A. Johnson Identification of Critical Illness-related Corticosteroid Insufficiency (CIRCI) in adult horses Graduate Students 9:15 Payal Agarwal INK4A/ARF Multifunctional Regulatory Tumor Suppressor Gene Locus in Canine Mammary Cancer 9:30 Allison M Bradbury Immunomodulatory Effects of AAV-mediated gene therapy in the treatment of GM2-Gangliosidosis 9:45 Lawrence A. Brown Comparison of three Magnetic Resonance Imaging sequences for measuring cartilage thickness in the canine stifle 10:00 Matthew Cannon Mitochondrial RNA import mechanisms - Identification of proteins selectively interacting with RNAs imported into mitochondria 10:15 Ghislaine Dujovne Effectiveness of a human contraceptive implant (Implanon®) on estrus -

CLASS of 2020 Student Handbook
CLASS OF 2020 Student Handbook AS OF JUNE 2016 CLASS OF 2020 Welcome Welcome to the College of Veterinary Medicine, and congratulations on your admission to the Class of 2020. You were selected from a large, talented field of ap- plicants; 102 students will matriculate in August. You are among a select few who have earned the privilege of a world-class education, at a college with internation- ally renowned faculty and facilities, a unique, innovative educational approach, and an unmatched network of academic support. In welcoming you to the College of Veterinary Medicine and Services has professionals dedicated to helping you in the ar- the veterinary profession, the faculty and staff of the College eas of academic and personal support, financial planning, and are committing to help you reach your career goals in veteri- career development. nary medicine. Years of thoughtful reflection on what is By coming to Cornell, you are making a commitment to your- known about learning and the demands of the veterinary pro- self, your families, your classmates, to the College and to your fessional practice, carefully-considered translations of state of future clients and patients. The very few students who start the art research in the biomedical but do not finish the DVM pro- and clinical sciences, and the on- gram represent a loss to the pro- going dedication of caring fac- fession and the college commu- ulty come together in the cur- nity. Thus, by accepting an offer riculum and learning environ- of admission, you accept the re- ment at Cornell. sponsibilities and privileges of a The faculty and staff want you member of this academic com- to succeed at Cornell and in munity, as well as the responsi- your professional life. -

Commencement Program
School of Veterinary Medicine Commencement May 21, 2021 1 SCHOOL OF VETERINARY MEDICINE Seventieth Commencement Ceremony FRIDAY, MAY 21, 2021 School of Veterinary Medicine Valley Hall Lawn University of California, Davis For more than 100 years, the University of California, Davis, has engaged in teaching, research and public service that matter to California and transform the world. Located close to the state capital, UC Davis has over 39,000 students, an annual research budget that exceeds $847 million, a comprehensive health system and 13 specialized research centers. The university offers interdisciplinary graduate study and more than 104 undergraduate majors in four colleges – Agricultural and Environmental Sciences, Biological Sciences, Engineering, and Letters and Science. It also houses six professional schools – Education, Law, Management, Medicine, Veterinary Medicine and the Betty Irene Moore School of Nursing. The School of Veterinary Medicine The UC Davis School of Veterinary Medicine provides teaching, research and service programs to benefit animal health, public health and environmental health. As the only veterinary school in the University of California system, we serve the entire state. The Doctor of Veterinary Medicine degree program is a four-year course of academic study and clinical training. Faculty research and the real-world cases of the William R. Pritchard Veterinary Medical Teaching Hospital are essential components of the program. Students enrich their learning through scientific projects, international exchange, volunteer activities and field experience. Since 1948, the School of Veterinary Medicine has launched the careers of more than 6,300 veterinarians and 960 Master of Preventive Veterinary Medicine professionals. More than 1,050 residents and dozens of master’s and doctoral students have completed training here. -

Typical CV Example
Mary K. Hondalus Curriculum Vitae 1. Academic History Name: Mary K. Hondalus Present rank: Associate Professor of Infectious Diseases Proportional Time Assignment: 50% Teaching, 40% Administrative, 10% service Tenure Status: tenured Graduate Faculty Status: Graduate Faculty, 2005- Present Highest Degree, Institution, and Date: Ph. D. Temple University School of Medicine, Philadelphia, PA, 1995 D.V.M. Michigan State University, East Lansing, MI, 1984 B. S. Michigan State University, East Lansing, MI, 1982 (degree conferred upon completion of two years of the veterinary curriculum) Academic Positions: 2015-present Director of Pre-Clinical Academic Affairs 2010-present Associate Professor Department of Infectious Diseases, College of Veterinary Medicine University of Georgia 2010-present Coordinator of the DVM-MPH Dual Degree Program 2004-2009 Assistant Professor Department of Infectious Diseases, College of Veterinary Medicine University of Georgia 2001-2004 Research Scientist, Department of Immunology and Infectious Diseases, Harvard School of Public Health, Boston MA 1999-2001 Research Associate, Department of Immunology and Infectious Diseases, Harvard School of Public Health, Boston, MA 1995-1999 Postdoctoral Associate, Howard Hughes Medical Institute at the Albert Einstein College of Medicine, Bronx NY Dec. 1991 Relief Large Animal Medicine Clinician, Tufts University Veterinary School, N. Grafton, MA 1988-1989 Clinical Fellow, Tufts University Veterinary School, N. Grafton, MA April 1988 Relief Large Animal Medicine Clinician, University of Prince Edward Island, Atlantic College of Veterinary Medicine, Charlottetown, P.E.I., Canada 1986-1988 Resident Large Animal Internal Medicine, Tufts Veterinary School, N. Grafton, MA Professional Positions: 1985 – 1986 Part-time Small Animal Veterinarian Northfield Animal Hospital, Northfield, NJ 1984 – 1986 Associate Equine Veterinarian, Leonard J. -

Sarah Anne Hamer
Sarah Anne Hamer Department of Veterinary Integrative Biosciences College of Veterinary Medicine and Biomedical Sciences Texas A&M University College Station, Texas, USA 77843-4458 Tel: +1-517-775-4360; Fax: +1-979-847-8981 Email: [email protected] Education Doctor of Veterinary Medicine, Michigan State University, East Lansing, MI 2011 Doctor of Philosophy, Michigan State University, East Lansing, MI 2010 - Dual-degree: Fisheries and Wildlife & Ecology, Evolutionary Biology, and Behavior - Fish and Wildlife Disease Ecology and Conservation Medicine Specialization Master of Science, University of Illinois, Champaign/Urbana, IL 2003 -Department of Natural Resources and Environmental Sciences -Wildlife Ecology Specialization Bachelor of Science, University of Illinois, Champaign/Urbana, IL 2001 -Major: Natural Resources and Environmental Sciences Professional Experience Assistant Professor, Texas A&M University 1/2012 - present Department of Veterinary Integrative Biosciences College of Veterinary Medicine and Biomedical Sciences Faculty in Ecology and Evolutionary Biology Program Faculty in Vector Biology Group Adjunct Assistant Professor, Michigan State University 11/2011 - present Department of Fisheries and Wildlife Veterinary Extern National Wildlife Health Center, Madison, WI 9/2010–10/2010 Assisted in field and laboratory research to detect a novel mosquito-borne Flavivirus in Wisconsin. Experiences included development and validation of enzyme-linked immunosorbent assays, trapping and blood-sampling of mammals, and reporting research findings. Mentor: Dr. Erik Hofmeister International Crane Foundation, Baraboo, WI 10/2010 Assisted in health checks of ten species of cranes. Duties included blood-sampling, clipping feathers, trimming bills, tube-feeding, post-surgical care, administering drugs, and data entry into MedArks program. Also assisted with hematology research. Mentor: Dr.