Skeletal Muscle – Inflammation
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Guidelines on the Diagnosis and Management of Pericardial
European Heart Journal (2004) Ã, 1–28 ESC Guidelines Guidelines on the Diagnosis and Management of Pericardial Diseases Full Text The Task Force on the Diagnosis and Management of Pericardial Diseases of the European Society of Cardiology Task Force members, Bernhard Maisch, Chairperson* (Germany), Petar M. Seferovic (Serbia and Montenegro), Arsen D. Ristic (Serbia and Montenegro), Raimund Erbel (Germany), Reiner Rienmuller€ (Austria), Yehuda Adler (Israel), Witold Z. Tomkowski (Poland), Gaetano Thiene (Italy), Magdi H. Yacoub (UK) ESC Committee for Practice Guidelines (CPG), Silvia G. Priori (Chairperson) (Italy), Maria Angeles Alonso Garcia (Spain), Jean-Jacques Blanc (France), Andrzej Budaj (Poland), Martin Cowie (UK), Veronica Dean (France), Jaap Deckers (The Netherlands), Enrique Fernandez Burgos (Spain), John Lekakis (Greece), Bertil Lindahl (Sweden), Gianfranco Mazzotta (Italy), Joa~o Morais (Portugal), Ali Oto (Turkey), Otto A. Smiseth (Norway) Document Reviewers, Gianfranco Mazzotta, CPG Review Coordinator (Italy), Jean Acar (France), Eloisa Arbustini (Italy), Anton E. Becker (The Netherlands), Giacomo Chiaranda (Italy), Yonathan Hasin (Israel), Rolf Jenni (Switzerland), Werner Klein (Austria), Irene Lang (Austria), Thomas F. Luscher€ (Switzerland), Fausto J. Pinto (Portugal), Ralph Shabetai (USA), Maarten L. Simoons (The Netherlands), Jordi Soler Soler (Spain), David H. Spodick (USA) Table of contents Constrictive pericarditis . 9 Pericardial cysts . 13 Preamble . 2 Specific forms of pericarditis . 13 Introduction. 2 Viral pericarditis . 13 Aetiology and classification of pericardial disease. 2 Bacterial pericarditis . 14 Pericardial syndromes . ..................... 2 Tuberculous pericarditis . 14 Congenital defects of the pericardium . 2 Pericarditis in renal failure . 16 Acute pericarditis . 2 Autoreactive pericarditis and pericardial Chronic pericarditis . 6 involvement in systemic autoimmune Recurrent pericarditis . 6 diseases . 16 Pericardial effusion and cardiac tamponade . -

NLRP3 Inflammasome Cutting Edge
Cutting Edge: Nitric Oxide Inhibits the NLRP3 Inflammasome Eduardo Hernandez-Cuellar, Kohsuke Tsuchiya, Hideki Hara, Rendong Fang, Shunsuke Sakai, Ikuo Kawamura, This information is current as Shizuo Akira and Masao Mitsuyama of September 25, 2021. J Immunol published online 24 October 2012 http://www.jimmunol.org/content/early/2012/10/24/jimmun ol.1202479 Downloaded from Supplementary http://www.jimmunol.org/content/suppl/2012/10/24/jimmunol.120247 Material 9.DC1 http://www.jimmunol.org/ Why The JI? Submit online. • Rapid Reviews! 30 days* from submission to initial decision • No Triage! Every submission reviewed by practicing scientists • Fast Publication! 4 weeks from acceptance to publication by guest on September 25, 2021 *average Subscription Information about subscribing to The Journal of Immunology is online at: http://jimmunol.org/subscription Permissions Submit copyright permission requests at: http://www.aai.org/About/Publications/JI/copyright.html Email Alerts Receive free email-alerts when new articles cite this article. Sign up at: http://jimmunol.org/alerts The Journal of Immunology is published twice each month by The American Association of Immunologists, Inc., 1451 Rockville Pike, Suite 650, Rockville, MD 20852 Copyright © 2012 by The American Association of Immunologists, Inc. All rights reserved. Print ISSN: 0022-1767 Online ISSN: 1550-6606. Published October 24, 2012, doi:10.4049/jimmunol.1202479 Cutting Edge: Nitric Oxide Inhibits the NLRP3 Inflammasome Eduardo Hernandez-Cuellar,*,† Kohsuke Tsuchiya,* Hideki Hara,* Rendong Fang,* Shunsuke Sakai,* Ikuo Kawamura,* Shizuo Akira,† and Masao Mitsuyama* Although the NLRP3 inflammasome plays a pivotal role in that NLRP3 inflammasome contributes to host defense against host defense, its uncontrolled activation is associated with microbialpathogens,excessiveactivationduetomutationsinthe inflammatory disorders, suggesting that regulation of the NLRP3 gene has been associated with a spectrum of autoin- inflammasome is important to prevent detrimental effects. -
Instant Notes: Immunology, Second Edition
Immunology Second Edition The INSTANT NOTES series Series Editor: B.D. Hames School of Biochemistry and Molecular Biology, University of Leeds, Leeds, UK Animal Biology 2nd edition Biochemistry 2nd edition Bioinformatics Chemistry for Biologists 2nd edition Developmental Biology Ecology 2nd edition Immunology 2nd edition Genetics 2nd edition Microbiology 2nd edition Molecular Biology 2nd edition Neuroscience Plant Biology Chemistry series Consulting Editor: Howard Stanbury Analytical Chemistry Inorganic Chemistry 2nd edition Medicinal Chemistry Organic Chemistry 2nd edition Physical Chemistry Psychology series Sub-series Editor: Hugh Wagner Dept of Psychology, University of Central Lancashire, Preston, UK Psychology Cognitive Psychology Forthcoming title Physiological Psychology Immunology Second Edition P.M. Lydyard Department of Immunology and Molecular Pathology, Royal Free and University College Medical School, University College London, London, UK A. Whelan Department of Immunology, Trinity College and St James’ Hospital, Dublin, Ireland and M.W. Fanger Department of Microbiology and Immunology, Dartmouth Medical School, Lebanon, New Hampshire, USA © Garland Science/BIOS Scientific Publishers Limited, 2004 First published 2000 This edition published in the Taylor & Francis e-Library, 2005. “To purchase your own copy of this or any of Taylor & Francis or Routledge’s collection of thousands of eBooks please go to www.eBookstore.tandf.co.uk.” Second edition published 2004 All rights reserved. No part of this book may be reproduced or -

Quantitative Studies of the Inflammatory Process in Fatal Viral Meningoencephalitis
Quantitative Studies of the Inflammatory Process in Fatal Viral Meningoencephalitis P. C. Doherty, MVSc, PhD The pathogenesis of acute meningoencephalitis induced in adult mice by in- travenous inoculation with Semliki Forest virus has been assessed by counting cells in cerebrospinal fluid (CSF). Meningitis was first apparent on day 4 and, by the time that animals were moribund 2 days later, each microliter of CSF contained in excess of 10,000 mononuclear cells. The following conclusions were made concerning this very considerable inflammatory response: a) Complete suppression of cellular infiltration makes no difference to the clinical disease. b) No correlation is apparent between inflammation and levels of circulating antibody. c) Participation of thymus-derived lymphocytes (T cells) is essential for full expression, though not for initiation, of cellular invasion. d) There is evidently no requirement for lymphocytes recently derived from thymus or for any humoral factor secreted by thymus tissue. e) T cells entering the recirculating pool more than 6 weeks or less than about 1 week prior to inoculation of virus are equally effective in promoting inflammation. f) The T cells apparently act directly by enhancing infiltration of other blood-borne mononuclears into the brain and CSF (Am J Pathol 73:607-622, 1973). A PROTECTIVE ROLE for the inflammatory response has been clearly demonstrated in several acute infectious diseases. The marked cellular infiltration occurring in ectromelia 1,2 and listeriosis 3'4 is associated with elimination of the parasite from the liver and sur- vival of the host. Inflammation is implicated in reducing virus titers in the nonfatal meningoencephalitis caused by intracerebral injec- tion of Sindbis virus." In a few conditions, however, the overall effect of the inflammatory process is deleterious. -
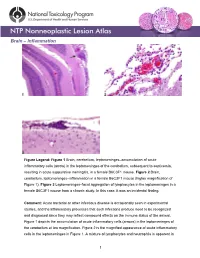
Brain – Inflammation
Brain – Inflammation Figure Legend: Figure 1 Brain, cerebellum, leptomeninges–accumulation of acute inflammatory cells (arrow) in the leptomeninges of the cerebellum, subsequent to septicemia, resulting in acute suppurative meningitis, in a female B6C3F1 mouse. Figure 2 Brain, cerebellum, leptomeninges–inflammation in a female B6C3F1 mouse (higher magnification of Figure 1). Figure 3 Leptomeninges–focal aggregation of lymphocytes in the leptomeninges in a female B6C3F1 mouse from a chronic study. In this case, it was an incidental finding. Comment: Acute bacterial or other infectious disease is occasionally seen in experimental studies, and the inflammatory processes that such infections produce need to be recognized and diagnosed since they may reflect compound effects on the immune status of the animal. Figure 1 depicts the accumulation of acute inflammatory cells (arrows) in the leptomeninges of the cerebellum at low magnification. Figure 2 is the magnified appearance of acute inflammatory cells in the leptomeninges in Figure 1. A mixture of lymphocytes and neutrophils is apparent in 1 Brain – Inflammation this exudate. Figure 3 shows the focal aggregation of lymphocytes in the leptomeninges of a mouse. In this case, it was an incidental finding. The occurrence of such a lesion may, however, indicate a response to a recent viral infection in the nervous system. Occasionally, a small, apparently inert cluster of mononuclear cells without any other reactive responses in the vicinity may be referred to as inflammatory infiltrate. However, the presence of neutrophils should alert the pathologist to conduct a diligent evaluation for other complementary signs of inflammation. In NTP studies, there are five standard categories of inflammation: acute, suppurative, chronic, chronic-active, and granulomatous. -

Immunology Lecture to Resume
Immunology Lecture to Resume D.HAMMOUDI.MD Part 1 :Generality The Invaders . Bacteria http://www.hhs.gov/asphep/presentation/images/bacteria.jpg Viruses parasites such as fungi, protista, & worms http://www.skidmore.edu/academics/biology/plant_bio/lab13.FUNGI.html http://www.sdnhm.org/exhibits/epidemic/teachers/background.html worm trichura.jpg Immunity: Two Intrinsic Defense Systems Innate (nonspecific) system responds quickly and consists of:[3 line of defense] First line of defense – skin and mucosa prevent entry of microorganisms Second line of defense – antimicrobial proteins, phagocytes, and other cells Inhibit spread of invaders throughout the body Inflammation is its most important mechanism •Adaptive (specific) defense system •Third line of defense – mounts attack against particular foreign substances Takes longer to react than the innate system Works in conjunction with the innate system Innate and Adaptive Defenses Figure 21.1 Outline of the Immune System Skin 1st Line of Defense Mucus Secretions Phagocytic Cells Innate Immunity Antimicrobial Proteins 2nd Line of Defense Other tissues which participate in inflammatory responses Lymphocytes Adaptive Immunity 3rd Line of Defense Antibodies Attenuated Viruses Killed Viruses Acquired Immunity Vaccines / Immunotherapies Toxoid Vaccines Component Vaccines Mechanical, Physical and Chemical Barriers What are the examples of Physiologic and Chemical Barriers at the skin and mucous membranes? Acid pH -- this also relates to the stomach Hydrolytic enzymes Proteolytic enzymes Interferon refers to a group of proteins that can help prevent the spread of viruses. There is one special one called gamma-interferon -- this one is a cytokine produced by TH cells. Complement is a term that refers to a group of serum proteins that are normally found "inactive" in the serum. -

Hormone Concentrations in Synovial Fluid of Patients with Rheumatoid Arthritis J
Hormone concentrations in synovial fluid of patients with rheumatoid arthritis J. Rovensky1, R. Kvetnansky2, Z. Radikova2, R. Imrich2, O. Greguska1, M. Vigas2, L. Macho2 1National Institute for Rheumatic Diseases, Piestany; 2Institute of Experimental Endocrinology, Slovak Academy of Sciences, Bratislava, Slovakia. Abstract Objective Alterations in local concentrations of hormones, affecting directly synovial cells, could be involved in the modulation of the rheumatic inflammatory processes. The aim of present study was to investigate the levels of select- ed hormones (steroids, peptide and thyroid hormones) in synovial fluid of knee joint of patients with rheumatoid arthritis (RA) and control individuals with non-rheumatic exudate (with osteoarthrosis, OA). Methods Thirty-eight patients, 22 female and 16 males, with rheumatoid arthritis (RA) and 12 subjects with osteoarthrosis (OA, control group, 6 females and 6 males) participated in the study. Concentrations of cortisol (CS), 17- estradiol (ES), dehydroepiandrosterone (DHEA), progesterone (PRG), aldosterone ALD), prolactin (PRL), insulin (INS), and C-peptide were determined by radioimmunoassay in synovial fluid. Insulin binding to isolated cell membrane of cells from synovial sediment was estimated by using radioiodine labeled insulin. In a group of patients (10 with RA and 4 with OS), the levels of free threeiodothyronine (FT3), TSH and growth hormone (GH) were also determined in synovial fluid. Results Increased levels of ES in synovial fluid of RA patients were observed, and higher differences were noted in men. TE concentrations were moderately elevated in synovial fluid of RA patients, however the ratio of ES/TE was significantly higher in male RA compared to OA patients. Higher levels of PRG, ALD and growth hormone were noted in synovial fluid of RA patients. -

Fibrin and Thrombosis in the Central Nervous System in Children with Particular Reference to Congenital Hydrocephalus
J Clin Pathol: first published as 10.1136/jcp.17.3.348 on 1 May 1964. Downloaded from J. clin. Path. (1964), 17, 348 Fibrin and thrombosis in the central nervous system in children with particular reference to congenital hydrocephalus JOHN L. EMERY From the Department ofPathology, The Children's Hospital, Sheffield While rare metabolic and degenerative diseases of scanty and a simple fibrin clot is rarely seen. the central nervous system in children are reported Surrounding the small veins there are often clear at great length, the much commoner diseases vacuolated spaces but there is no evidence of relating to vascular incidents are rarely discussed. inflammation of the vein wall or of reaction in the Only those aspects of disease of the central surrounding glia. Occasionally there are small local nervous system related to thrombosis and the haemorrhages. There are three clinical states in deposition of fibrin will be discussed here; diseases which thrombi are most likely to be encountered. related to general vasculitis and aneurysm are omitted. The conditions discussed here fall into two SEVERE DEHYDRATION AND INANITION This situation main categories: first, the presence of thrombosis in is liable to occur associated with vomiting or small blood vessels or in the large superficial veins diarrhoea. There is frequently massive thrombosis, draining the brain, and second, the deposition of often involving the longitudinal sinus and well fibrin either in the ventricles or on the membranes known in the past as 'marantic thrombosis'. overlying the brain in association with thrombotic The second condition is liable to occur in young lesions, haemorrhage, or infiltration. -

Identification of Malignant Mesothelioma Risk Factors Through Association Rule Mining
Preprints (www.preprints.org) | NOT PEER-REVIEWED | Posted: 10 November 2019 doi:10.20944/preprints201911.0117.v1 Article Identification of Malignant Mesothelioma Risk Factors through Association Rule Mining Talha Mahboob Alam Computer Science and Engineering Department University of Engineering and Technology Lahore, Pakistan [email protected] Abstract: Malignant mesothelioma is a rare proliferative cancer that develops in the thin layer of tissues surrounding the lungs. Malignant mesothelioma is associated with an extremely poor prognosis and the majority of patients do not show symptoms. The epidemiology of mesothelioma is important for the identification of disease. The primary aim of this study is to explore the risk factors associated with mesothelioma. The dataset consists of healthy and mesothelioma patients but only mesothelioma patients were selected for the identification of symptoms. The raw data set has been pre-processed and then the Apriori method was utilized for association rules with various configurations. The pre-processing task involved the removal of duplicated and irrelevant attributes, balanced the dataset, numerical to the nominal conversion of attributes in the dataset and creating the association rules in the dataset. Strong associations of disease’s factors; asbestos exposure, duration of asbestos exposure, duration of symptoms, erythrocyte sedimentation rate and Pleural to serum LDH ratio determined via Apriori algorithm. The identification of risk factors associated with mesothelioma may prevent patients from going into the high danger of the disease. This will also help to control the comorbidities associated with mesothelioma which are cardiovascular diseases, cancer-related emotional distress, diabetes, anemia, and hypothyroidism. Keywords: Malignant mesothelioma, Epidemiology, Association rule mining, Apriori method, Imbalanced dataset I. -

86116-Meningitis and Complications OSBORN.Pdf
MENINGITIS… INFECTIOUS MENINGITIS And Its Complications: • Clinical presentation • Headache ± fever, nuchal rigidity Radiologic-Pathologic Correlations • AMS (acute febrile encephalopathy) • Etiologies vary • Pyogenic • Viral • Granulomatous • Pathology similar • Dense, purulent exudate • Covers pial surface of brain • Often fills subarachnoid spaces • Variable extension into brain via PVSs Anne G. Osborn, M.D. • ± Involvement of blood vessels ( vasculitis) ACUTE MENINGITIS CT OF ACUTE MENINGITIS Generic Imaging Patterns • May be normal early! Mild ventricular enlargement + “featureless” brain Most common = pia-subarachnoid Less common = focal or diffuse dura- • Findings space exudates (acute > chronic) arachnoid thickening (chronic > acute) • Nonspecific hydrocephalus • Sulcal/cisternal effacement • Pia/subarachnoid enhancement “Effaced” sulci on NECT; enhancing on CECT MR OF MENINGITIS MR OF MENINGITIS T1WI FLAIR Sulci “bright”on FLAIR, ± slightly ↑ • General features Pus fills in sulci • “Dirty” CSF • Iso- (“gray-ish” CSF) on T1WI • Hyperintense on T2WI (hard to see!) • Hyperintense on FLAIR (nonspecific) • Sulcal-cisternal enhancement on T1C+ • Post-contrast FLAIR • More sensitive than T1C+FS! • Other findings • ± Foci of restricted diffusion • Sulci, parenchyma T1C+FS Pus in sulci restricts on DWI (these aren’t strokes!) Page 1 DDX FLAIR HYPERINTENSE CSF COMPLICATIONS OF MENINGITIS Baseline MR • Altered CSF flow, resorption → • Subarachnoid hemorrhage • Hydrocephalus • Meningitis • Often earliest (sometimes only) finding! • Leptomeningeal -

Pathology Inflammation Lectures
INFLAMMATION Definition • Inflammation is a defensive process that a living body initiates against local tissue damage. It takes the form of a complex reaction of blood vessels, certain plasma components and blood cells, and cellular and structural components of connective tissue. • Terms ending in the suffix “–itis” denote inflammation. Etiology of Inflammation • Physical agents: • extreme temperatures, electric shock, radiation, mechanical injures, etc. • Chemical agents: • Products of metabolism, acids, alkalis, drugs, tissue necrosis • Biological agents: • Microorganisms (bacteria, viruses, fungi), parasites (helmints, insects), immune cells and complexes What MUST be known! • Understanding of pathogenic mechanisms is important diagnose inflammatory processes and diseases • Inflammation exists until is eliminated the etiological factor and are inactivated inflammatory mediators • Inflammation is potentially dangerous and should be restricted • Therapy should be etiopathogenic Clinical presentation (rubor, tumor) Inflammatory phases: • Alteration – damage (dystrophy and necrosis) • Exudation – the reaction of microcirculation, formation of liquid exudate, migration of leukocytes and phagocytosis • Proliferation - proliferation of cell of hematogenous (macrophages, lymphocytes) and histiogenous (fibroblasts) nature The classification of inflammation • According to the course: • acute, • subacute, • chronic • According to the predominant phase: • alterative, • exudative, • proliferative (productive) • According to the causative factors: -

Treatment of Gingival Enlargement Shruti Bhatnagar
Chapter Treatment of Gingival Enlargement Shruti Bhatnagar Abstract Gingival enlargement or overgrowth is a common disease of gingiva. The causative factors may range from inflammation due to local factors to conditioned enlargement and neoplastic enlargements. They commonly present as bulbous interdental gingival, diffuse swelling of gingival. Due to the unaesthetic appearance of the overgrown gingiva, treatment becomes inevitable. This results in excision of overgrowth known as gingivectomy. The first gingivectomy procedure was explained by Robicsek in 1884 and later by Zentler (1918). Grant (1979) defined gingivectomy as excision of soft tissue wall of pathologic periodontal pocket. Gingivectomy procedures can be done by means of scalpel, laser, electrosurgery and chemosurgery. The ultimate result remains the same indifferent of the method used. However the amount of remaining keratin- ized gingival and esthetic appearance is of supreme importance. Keywords: gingival enlargement, gingival overgrowth, gingival hyperplasia, gingivectomy, gingival diseases, anticonvulsants, abscess 1. Introduction Gingival enlargement is a common clinical problem, usually associated with specific conditions. This condition finds a unique place in literature, because it has been associated with a variety of local and systemic factors. Enlargement of any part, tissue or organ in the body may be attributable to one or more of the following pathological processes [1]: Cellular hypertrophy: defined as an increase in the size of a part due to an increase in the size of the individual cells comprising that part. Cellular hyperplasia: increase in size due to an increase in the absolute number of cells, though cell size is not altered. Fibrosis: an accumulation of collagenous connective tissue which is classically characterized by relative acellularity.