The Specificity of Dimethylbenzylrifampicin As An
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Rheumatoid Arthritis a Virus Disease?
J Clin Pathol: first published as 10.1136/jcp.s3-12.1.132 on 1 January 1978. Downloaded from J. clin. Path., 31, Suppl. (Roy. Coll. Path.), 12, 132-143 Inflammation and fibrosis Rheumatoid arthritis a virus disease? A. M. DENMAN From the Division ofImmunological Medicine, Clinical Research Centre, Harrow, Middlesex We now understand many of the immunopatho- arteritis with perivascular infiltrates of chronic logical processes that damage joints and other inflammatory cells, and aberrant immune responses. structures in rheumatoid arthritis and diffuse con- Secondly, virus-infected cells initiate the whole nective tissue diseases. Unfortunately, our progress spectrum of inflammatory and immune reactions in this direction is matched by an equal failure to which characterise the human disorders. These identify the causes of all but a few of these disorders. include complement activation by the classical and Nevertheless, the stimuli which initiate these diseases alternative pathways, which in turn initiate such are probably commonly encountered and tissue processes as the immediate hypersensitivity reaction, damage results when these immunopathological platelet aggregation, and the chemotaxis of granulo- processes are abnormal in intensity and duration- cytes and mononuclear phagocytes. Furthermore, in other words, disease follows an unusual host virus-infected cells attract antibody either through reaction to a variety of environmental factors. This virus-coded antigens on the cell membrane or principle is well illustrated by the degenerative because infected cells commonly carry receptors for copyright. disease of the central nervous system, subacute the Fc portion of the immunoglobulin molecule. sclerosing panencephalitis, which ensues in in- These cells also attract a cellular response in the dividuals who are unable to control the growth form of both specifically sensitised T lymphocytes and dissemination of measles virus. -

Barriers to Adoption of GM Crops
Iowa State University Capstones, Theses and Creative Components Dissertations Fall 2021 Barriers to Adoption of GM Crops Madeline Esquivel Follow this and additional works at: https://lib.dr.iastate.edu/creativecomponents Part of the Agricultural Education Commons Recommended Citation Esquivel, Madeline, "Barriers to Adoption of GM Crops" (2021). Creative Components. 731. https://lib.dr.iastate.edu/creativecomponents/731 This Creative Component is brought to you for free and open access by the Iowa State University Capstones, Theses and Dissertations at Iowa State University Digital Repository. It has been accepted for inclusion in Creative Components by an authorized administrator of Iowa State University Digital Repository. For more information, please contact [email protected]. Barriers to Adoption of GM Crops By Madeline M. Esquivel A Creative Component submitted to the Graduate Faculty in partial fulfillment of the requirements for the degree of MASTER OF SCIENCE Major: Plant Breeding Program of Study Committee: Walter Suza, Major Professor Thomas Lübberstedt Iowa State University Ames, Iowa 2021 1 Contents 1. Introduction ................................................................................................................................. 3 2. What is a Genetically Modified Organism?................................................................................ 9 2.1 The Development of Modern Varieties and Genetically Modified Crops .......................... 10 2.2 GM vs Traditional Breeding: How Are GM Crops Produced? -

Prostate Cancer Chemoprevention Agents Exhibit Selective Activity Against Early Stage Prostate Cancer Cells
Prostate Cancer and Prostatic Diseases (2001) 4, 81±91 ß 2001 Nature Publishing Group All rights reserved 1365±7852/01 $15.00 www.nature.com/pcan Prostate cancer chemoprevention agents exhibit selective activity against early stage prostate cancer cells YQ Liu1, E Kyle2, S Patel2, F Housseau3, F Hakim2, R Lieberman4, M Pins5, MV Blagosklonny2 & RC Bergan1* 1Division of Hematology/Oncology, Department of Medicine, Northwest University Medical School and the Robert H. Lurie Cancer Center of Northwestern University, Chicago, USA; 2Medicine Branch, National Cancer Institute, National Institutes of Health, Bethesda, MD, National Institutes, National Institutes USA; 3Surgery Branch, National Cancer Institute, National Institutes of Health, Bethesda, MD, USA; 4Division of Cancer Prevention, National Cancer Institute, National Institutes of Health, Bethesda, MD, USA; and 5Department of Pathology, Northwestern University Medical School and the Robert H. Lurie Cancer Center of Northwestern University, Chicago, USA Preclinical models for the identi®cation of prostate cancer chemoprevention agents are lacking. Based upon the notion that clinically useful chemoprevention agents should exhibit selective activity against early stage disease, studies were undertaken to assess whether chemoprevention agents selectively inhibited the growth of early stage prostate cancer, as compared to late stage cancer. First, a series of cell and molecular studies were performed, which, when taken together, validated the use of a panel of prostate cell lines as a model of the different stages of carcinogenesis. Next, therapeutic responsiveness to ten different cytotoxic or chemoprevention agents was evaluated. Chemoprevention agents exhibited selective activity against normal and early transformed prostate tissue, whereas cytotoxic agents were non-speci®c. Selective activity against early versus advanced prostate cancer cells is identi®ed as a potential screening method for chemoprevention agents. -

Viral Transformation
Proc. Natl. Acad. Sci. USA Vol. 75, No. 5, pp. 2473-2477, May 1978 Microbiology New region of the simian virus 40 genome required for efficient viral transformation (viable deletion mutants/soft agar assay/small t antigen) NOEL BOUCK*, NADINE BEALES*, THOMAS SHENK , PAUL BERGf, AND GIAMPIERO DI MAYORCA* * Department of Microbiology, University of Illinois at the Medical Center, Chicago, Illinois 60680; t Department of Microbiology, University of Connecticut Health Center, Farmington, Connecticut 06032; and * Department of Biochemistry, Stanford University Medical Center, Stanford, California 94305 Contributed by Paul Berg, February 13,1978 ABSTRACT Viable mutants of simian virus 40 with dele- deletions in the proximal part of the early region affect a dif- tions in three regions of the virus genome (map coordinates ferent function than the one altered in the ts A mutants. 0.21-0.17, 0.59-0.54, and 0.67-0.74) have been tested for their ability to transform rat fibroblasts to anchorage independence. MATERIALS AND METHODS Only those mutants whose deletions occur between 0.59 and 0.55 in the proximal part of the early region are defective in trans- Cells and Viruses. Fischer rat line Fill (15), kindly sent to forming ability. The most severely defective of these transform us by A. Freeman, and passaged less than 25 times, was used with less than one-hundredth the efficiency of wild type. They for most transformation experiments. Chinese hamster lung retain their defect when tested in Chinese hamster lung cells cells, CHL (7), were obtained from R. Martin. The monkey and when infection is initiated with viral DNA instead of intact line BSC-1 was used virions. -

Wo 2008/048344 A2
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) CORRECTED VERSION (19) World Intellectual Property Organization International Bureau (43) International Publication Date (10) International Publication Number 24 April 2008 (24.04.2008) PCT WO 2008/048344 A2 (51) International Patent Classification: (81) Designated States (unless otherwise indicated, for every C12N 15/34 (2006.01) C12N 15/10 (2006.01) kind of national protection available): AE, AG, AL, AM, AT, AU, AZ, BA, BB, BG, BR, BW, BY, BZ, CA, CH, CN, (21) International Application Number: CO, CR, CU, CZ, DE, DK, DM, DZ, EC, EE, EG, ES, FI, PCT/US2007/003948 GB, GD, GE, GH, GM, GT, HN, HR, HU, ID, IL, IN, IS, (22) International Filing Date: JP, KE, KG, KM, KN, KP, KR, KZ, LA, LC, LK, LR, LS, 13 February 2007 (13.02.2007) LT, LU, LV,LY, MA, MD, ME, MG, MK, MN, MW, MX, MY, MZ, NA, NG, NI, NO, NZ, OM, PG, PH, PL, PT, RO, (25) Filing Language: English RS, RU, SC, SD, SE, SG, SK, SL, SM, SV, SY, TJ, TM, TN, TR, TT, TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, ZW (26) Publication Language: English (30) Priority Data: (84) Designated States (unless otherwise indicated, for every 60/773,255 13 February 2006 (13.02.2006) US kind of regional protection available): ARIPO (BW, GH, GM, KE, LS, MW, MZ, NA, SD, SL, SZ, TZ, UG, ZM, (71) Applicant (for all designated States except US): FRAUN- ZW), Eurasian (AM, AZ, BY, KG, KZ, MD, RU, TJ, TM), HOFER USA, INC. -

903, 943 Ff., 958 F., 1025 – Organismic 686 F
1107 Index 14-3-3 108, 349 ff., 470 ff., 500, 529, 764 ff., AGC kinase 464 ff. 950 ff., 1057 agents – targeted 1079 a aging 17AAG (17-allylamino-17-demethoxygeld- – cellular 686 anamycin) 903, 943 ff., 958 f., 1025 – organismic 686 f. A20 108 AHA1 940 aberration AIDS 3, 67 – chromosomal 807 AIF (apoptosis inducing factor) 5, 205 ff., ACAMP, see apoptotic cell-associated 225 ff., 233 ff., 760, 850, 948 ff., 1059 molecular pattern – apoptotic function 238 acetylation 1015 ff. – cancer 249 acid sphingomyelinase (aSMase) 104, 997 f. – DNA fragmentation 246 actinomycin D 854 – down regulation 239 activation-induced cell death (AICD) – expression 234 10, 96 ff. – homolog 237 activin 495, 1099 – isoform 236 ACTR 1017 – release 241 acute myeloid leukemia (AML) 71, 419, – structure 234 917, 1018 AIF-homologous mitochondrion-associated acute promyelocytic leukemia 678, 1017 f. inducer of cell death (AMID), acyl-CoA-binding protein (ACBP) 210 see also p53-responsive gene 3 207, 237 adaptor protein (AP) AIF-like (AIFL) 207, 237 – AP-1 603 Akt, see protein kinase B – AP-2 603 aliphatic acids 1019 – AP-3 603 alkylating agent 799 – AP-4 603 alkylation therapy 842 adenine nucleotide translocator (ANT) alkyllysophospholipids (ALP) 1068 241, 286, 352, 578 alkylphosphocholines 1068 adenomatous polyposis alkyltransferase 799 – coli (APC) 176, 478 Alzheimerls disease 140 – familiarly adenomatous polyposis (FAP) aminopeptidase N (CD13) 112 syndrome 1073 aminophospholipid translocase 586 f. adenovirus amplification 164, 894, 973, 1018 – adenoviral gene transfer 903, 1035 AN-9 1026 – adenoviral vector-based system 852 anaplastic large cell lymphoma 678 – conditionally replicative 1035 androgen receptor (AR) 163 ff. -

Selective Inhibition of Growth of Transformed Cells by Protease Inhibitors* (Contact Inhibition/Mouse/Hamster)
Proc. Nat. Acad. Sci. USA Vol. 69, No. 12, pp. 3825-3827, December 1972 Selective Inhibition of Growth of Transformed Cells by Protease Inhibitors* (contact inhibition/mouse/hamster) HANS PETER SCHNEBLIt AND MAX M. BURGER$ t Friedrich Miescher-Institute, P.O. Box 273, CH4002 Basel, Switzerland; and Princeton University, Department of Biochemical Sciences, Princeton, New Jersey 08540 Communicated by Vladimir Prelog, September 26, 1972 ABSTRACT Five protease inhibitors with different All cells were grown in Dulbecco's modified Eagle's Medium modes of action were found to reduce the growth of trans- (Gibco No. H-16) containing 10% fetal-calf serum (Micro- formed mouse (Py3T3, SV3T3, and 3T12) and hamster (PyBHK) cells. Some of these inhibitors caused the trans- biological Assoc., Bethesda, Md.). Stocks were passaged two formed cells to cease growth at saturation densities charac- to three times weekly, and care was taken to keep normal teristic for nontransformed cells. cells at low densities. Cells were checked for pleuropneumonia- The protease inhibitors were strikingly selective with like organisms regularly and were free of contamination regard to the transformed cells; they had essentially no the effect on the growth of the nontransformed cells. From throughout study. this result, it is concluded that the inhibitors block a pro- Cell growth curves in the presence or absence of protease tease-like activity that is required for the unrestrained inhibitors were obtained as follows: the cells were plated in growth of transformed cells. 3.5-cm petri dishes (Falcon) 48 hr before addition of the in- The inhibitors exerted their effect directly on the cells; hibitors. -
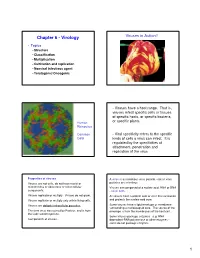
Chapter 6 - Virology Viruses in Action!!
Chapter 6 - Virology Viruses in Action!! • Topics – Structure – Classification – Multiplication – Cultivation and replication – Nonviral infectious agent – Teratogenic/Oncogenic - Viruses have a host range. That is, viruses infect specific cells or tissues of specific hosts, or specific bacteria, Human or specific plants. Rhinovirus Common - Viral specificity refers to the specific Cold kinds of cells a virus can infect. It is regulated by the specificities of attachment, penetration and replication of the virus Properties of viruses A virion is an infectious virus particle - not all virus Viruses are not cells, do not have nuclei or particles are infectious mitochondria or ribosomes or other cellular Viruses are composed of a nucleic acid, RNA or DNA components. - never both . Viruses replicate or multiply. Viruses do not grow. All viruses have a protein coat or shell that surrounds Viruses replicate or multiply only within living cells. and protects the nucleic acid core. Viruses are obligate intracellular parasites . Some viruses have a lipid envelope or membrane surrounding a nucleocapsid core. The source of the The term virus was coined by Pasteur, and is from envelope is from the membranes of the host cell. the Latin word for poison. Some viruses package enzymes - e.g. RNA- Components of viruses - dependent-RNA polymerase or other enzymes - some do not package enzymes 1 Size comparison of viruses - how big are they? Structure • Size and morphology • Capsid • Envelope • Complex • Nucleic acid Mycoplasma? There are two major structures -

UNIT-I DNA: Definition, Structure & Discovery DNA
SRINIVASAN ARTS AND SCIENCE COLLEGE (CO-ED) PERAMBALUlR - 621212 DEPARTMENT OF BIOTECHNOLOGY CLASS : I M.SC., BIOTECHNOLOGY SUBJECT: rDNA TECHNOLOGY SUB CODE : P16BT21 UNIT-I DNA: Definition, Structure & Discovery DNA The structure of the DNA double helix. The atoms in the structure are colour-coded by element and the detailed structures of two base pairs are shown in the bottom right. The structure of part of a DNA double helix Deoxyribonucleic acid is a molecule composed of two polynucleotide chains that coil around each other to form a double helix carrying genetic instructions for the development, functioning, growth and reproduction of all known organisms and many viruses. DNA and ribonucleic acid (RNA) are nucleic acids. Alongside proteins, lipids and complex carbohydrates (polysaccharides), nucleic acids are one of the four major types of macromolecules that are essential for all known forms of life. The two DNA strands are known as polynucleotides as they are composed of simpler monomeric units called nucleotides. Each nucleotide is composed of one of four nitrogen-containing nucleobases (cytosine [C], guanine [G], adenine [A] or thymine [T]), a sugar called deoxyribose, and a phosphate group. The nucleotides are joined to one another in a chain by covalent bonds (known as the phospho-diester linkage) between the sugar of one nucleotide and the phosphate of the next, resulting in an alternating sugar-phosphate backbone. The nitrogenous bases of the two separate polynucleotide strands are bound together, according to base pairing rules (A with T and C with G), with hydrogen bonds to make double- stranded DNA. The complementary nitrogenous bases are divided into two groups, pyrimidines and purines. -

Copy of Growing Into Their
Growing into their Own? Plant Molecular Farming and the Pursuit of Biotechnological Sovereignty for Lower and Middle Income Countries. Ana Christine Zeghibe Submitted in Partial Fulfillment of the Prerequisite for Honors In Biological Sciences Under the advisement of Martina Koniger, Ph.D. Wellesley College May 2021 © 2021 Ana Zeghibe Acknowledgements I am extraordinarily grateful for the support, kindness and guidance of my major and thesis advisor Professor Martina Koniger. The strong plant biology influence on an otherwise biotechnologically and medically oriented thesis was largely inspired by her own expertise in chloroplasts. When coming to this thesis, I knew I wanted to write something that was not simply a research paper, but a synthesis of ideas and observations about science and its interactions with the world beyond the laboratory that I had made over my 4 years at Wellesley. Thank you, as always, for allowing me to develop this unusual project, for keeping me sane through the many pitfalls and trials of writing, and for making me fall in love with science communication and plant biology. Thank you to my committee member Professor Natali Valdez for supporting and guiding my analyses and thoughts on the societal implications of plant molecular pharming. Professor Valdez's classes and work in Feminist Science and Technology Studies strongly influenced the later chapters of this thesis, helping me to think about the hopeful possibilities and critical realities for humanitarian applications of this unusual biotechnology. I am extremely thankful for her critiques of my argument and writing, her insight into the existing literature on biotechnological sovereignty and her passion for this project's potential. -

Problem of Genetically Modified Foods Safety: a Toxicologist’S View
REVIEWS UDC 604.6 doi: 10.15407/biotech9.01.007 PROBLEM OF GENETICALLY MODIFIED FOODS SAFETY: A TOXICOLOGIST’S VIEW E. L. LEVITSKY Palladin Institute of Biochemistry of the National Academy of Sciences of Ukraine, Kiyv E-mail: [email protected] Received 20.10.2015 This study aimed to analyze the published literature regarding the problem of safety of consuming food products containing genetically modified organisms. Genetically modified food products are given a brief definition, purpose and methods of their production are described, and the pro- and contra- arguments for their consumption are presented. The discussion is mostly focused on results of evaluating possible toxicity of such foods and their safety for macroorganism using traditional methods of toxicological analysis. Test results for long-term toxic effects, namely allergenicity, carcinogenicity, reproductive toxicity, and the possibility of mutagenic effects of these food products on the human body and the intestinal microflora are discussed separately. These data are based on the current understanding of the laws of the penetration and functioning of foreign genetic material outside the body, its entry and the possibility of integration into the genome during intake of foods manufactured by genetic engineering. The basic principles of the toxicological and hygienic regulation of these food products are also considered. An analysis of published experimental results allowed to draw a general conclusion about the absence of reliable scientific information indicating the presence of the toxic properties of genetically modified foods, and therefore of credible evidence of the dangers of consuming for humans and pets. Key words: genetically modified foods, toxicity, safety. -

Transformation and Oncogenesis Lecture 18 Biology 3310/4310 Virology Spring 2017
Transformation and Oncogenesis Lecture 18 Biology 3310/4310 Virology Spring 2017 Cause and effect, means and ends, seed and fruit, cannot be severed; for the effect already blooms in the cause, the end pre- exists in the means, the fruit in the seed. RALPH WALDO EMERSON Transformation Virology Lectures 2017 • Prof. Vincent Racaniello • Columbia University Principles of Virology, ASM Press The puzzling properties of transformed cells in the laboratory • Immortal: Grow indefinitely (HeLa) • Loss of anchorage dependence • Loss of contact inhibition • Colony formation in semi-solid media • Decreased requirements for growth factors (serum) Virology Lectures 2017 • Prof. Vincent Racaniello • Columbia University Oncogenesis • Development of cancer - Tumor: swelling caused by abnormal growth of tissue, benign or malignant • Cancer is a genetic disease • 8.2 million deaths/yr developed countries • Mutations (~12) affect signal transduction pathways that govern cell proliferation, survival, determination of cell fate, maintenance of genome integrity • Mutations may be inherited, caused by DNA damage, environmental carcinogens, infectious agents including viruses Virology Lectures 2017 • Prof. Vincent Racaniello • Columbia University Transformation and oncogenesis are distinct Requires additional genetic changes May not be oncogenic • Studying virus-transformed cells provides insight into molecular events that establish oncogenic potential • No virus can do it all Virology Lectures 2017 • Prof. Vincent Racaniello • Columbia University Human cancer viruses