The Coloration
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Annexes Table of Content
Annexes Table of content Annexes ................................................................................................................... 1 Table of content ........................................................................................................ 1 List of tables ............................................................................................................. 6 List of figures ............................................................................................................ 8 Annex A: Manufacture and uses ................................................................................. 10 A.1. Manufacture, import and export ........................................................................... 10 A.1.1. Manufacture, import and export of textiles and leather ........................................ 10 A.1.2. Estimated volumes on of the chemicals used in textile and leather articles ............. 12 A.2. Uses ................................................................................................................. 13 A.2.1. The use of chemicals in textile and leather processing ......................................... 13 A.2.1.1. Textile processing ................................................................................... 13 A.2.1.2. Leather processing ................................................................................. 19 A.2.1.3. Textile and leather formulations ............................................................... 20 A.2.1.4. Chemicals used in -

"3-47-2. 2,720,501 United States Patent Office Patented Oct
Oct. 11, 1955 R. T. VAN NESS 2,720,501 AQUEOUS CONDENSATION PROCESS FOR THE PREPARATION OF POLY WINYL ACETAL RESINS Filed Aug. 10, 1954 ALDEHYDE-f f 27 INVENTOR Robert Terry Van Ness "3-47-2. 2,720,501 United States Patent Office Patented Oct. 11, 1955 2 alcohol groups, and the remainder is polyvinyl butyral. In the attached drawing there is illustrated in a sche 2,720,501 matic fashion a flow sheet representing the process of AQUEOUS CONDENSATIGN PROCESS FOR THE this invention. A solution of polyvinyl alcohol in water PREPARATION OF POLYWNYLACETAL RESNS is prepared in vessel which is fitted with a means for agitating and a means for heating the contents of the Robert Terry Van Ness, Wilmington, Del, assignor to vessel. Polyvinyl alcohol from a storage vessel 3 is E. I. du Pont de Nemours and Company, Wimington, added to vessel 1 along with sufficient cold water at a Del, a corporation of Delaware temperature from about 20° C. to 30° C. to form a sus Application August 10, 1954, Serial No. 448,876 10 pension containing about 7 to about 15% solid polyvinyl alcohol. Polyvinyl alcohol is prepared by hydrolyzing 4 Claims. (CI. 260-73) polyvinyl acetate in the presence of an alkaline catalyst or an acid catalyst. Depending upon the polyvinyl ace tate used, the type of hydrolysis catalyst, and the de This invention relates to the preparation of polyvinyl 5 gree to which the hydrolysis is completed, many varieties acetal resins and, more particularly, it relates to a proc of polyvinyl alcohol may be prepared. -

US5147908.Pdf
|||||||||||||||| USOO5147908A United States Patent (19) 11 Patent Number: 5,147,908 Floyd et al. 45) Date of Patent: Sep. 15, 1992 (54) CATIONICPOLYVINYL ALCOHOL BINDER 4,405,375 9/1983 Gibson et al........................ 106/277 ADDITIVE 4,461,858 7/1984 Adelmann ........ ... 524/503 4,528,316 7/1985 Soerens ........ ... 524/503 75) Inventors: William C. Floyd, Chester; Louis R. 4,537,807 8/1985 Chan et al. ............................ 428/74 Dragner, Rock Hill, both of S.C. 4,816,540 3/1989 Onishi .......... ... 527/300 4,837,087 6/1989 Floyd et al. ......................... 428/51 73 Assignee: Sequa Chemicals Inc., Chester, S.C. 4,888,386 12/1989 Huang et al. ... ... 524/503 X (21) Appl. No.: 587,012 4,954,577 9/1990 Dinwald et al. ............... 524/503 X 22 Filed: Sep. 24, 1990 OTHER PUBLICATIONS 51) Int. Cl. ................................................ CO8L 3/04 Zunker, David W.; "Incorporating The Benefits Of 52 U.S. Cl. ........................................ 524/49; 524/55; Polyvinyl Alcohol At the Wet-End Of Papermaking'; 524/503; 525/57; 525/58 1982 Papermakers Conference. 58 Field of Search ..................... 524/503, 49, 55, 57, 524/58 Primary Examiner-Joseph L. Schofer Assistant Examiner-J. M. Reddick 56) References Cited Attorney, Agent, or Firm-Mitchell D. Bittman U.S. PATENT DOCUMENTS 57 ABSTRACT 3,573,236 3/1971 Barlow .....................r 260/7 3,597,313 8/1971 Williams et al. ... ... 162/67 A cationic polyvinyl alcohol binder additive is prepared 3,600,342 8/97 Nickerson et al. ................... 260/17 suitable for addition in the wet-end of a paper making 3,700,61 0/1972 Nickerson et al. -

Determination of Polymer Stabilizers
FLUORESCENCE APPLICATIONS ANALYSIS OF POLYMER STABILIZERS BY FLUORESCENCE SPECTROSCOPY USING THE MODEL LS-50 WITH THE FRONT SURFACE ACCESSORY ABSTRACT Quantitative analysis of a polymer UV stabilizer has been carried out using the PerkinElmer Model LS-50 Luminescence Spectrometer (L225-0105) fitted with the front surface accessory (5212-3130). The relationship between fluorescence emission and stabilizer concentration was found to be linear over the range observed for both native and extruded polymer samples. INTRODUCTION Many polymers such as polypropylene, polyethylene and polyvinyl chloride contain stabilizers to increase resistance to ultraviolet light-induced degradation. These additives can be relatively expensive, and the concentration required for effective UV stabilization can be quite critical. It is therefore desirable to monitor the concentration of the stabilizer in the parent polymer rather than relying on the calculation of the stabilizer dosing concentration based on the concentrations of the starting materials. This method allows for checking of homogeneity as well as the absolute concentration of the stabilizer. METHOD Polymer samples were supplied as native and extruded clear sheets. These were cut into 4 cm square sections and inserted into the front surface accessory using a piece of non-fluorescent card to keep the samples flat against the accessory window. Concentration of stabilizer was given as 0.5, 1.0, 1.5, 2.0, 2.5 and 3.0 mole %. RESULTS Data was collected as emission spectra, 390 nm - 550 nm, excitation wavelength 370 nm, slits 10/10 nm, scan speed 120 nm/min. The built-in emission attenuator (4 %T) was selected to decrease the assay sensitivity, as the samples were initially too strong for the extremely sensitive LS-50. -

An Analysis of the Mechanisms and Efficacy of Three Liquid Chemical Soil Stabilizers
Technical Report Documentation Page 1. Report No. 1993-1 2. Government 3. Recipient’s Catalog No. FHWA/TX-03/1993-1 (Volume 1) Accession No. 4. Title and Subtitle 5. Report Date AN ANALYSIS OF THE MECHANISMS AND EFFICACY May 2003 OF THREE LIQUID CHEMICAL SOIL STABILIZERS: VOLUME 1 7. Author(s) 6. Performing Organization Code Alan F. Rauch, Lynn E. Katz, and Howard M. Liljestrand 8. Performing Organization Report No. 1993-1 9. Performing Organization Name and Address 10. Work Unit No. (TRAIS) Center for Transportation Research The University of Texas at Austin 11. Contract or Grant No. 3208 Red River, Suite 200 Research Project 7-1993 Austin, TX 78705-2650 12. Sponsoring Agency Name and Address 13. Type of Report and Period Covered Texas Department of Transportation Research Report Research and Technology Implementation Office 14. Sponsoring Agency Code P.O. Box 5080 Austin, TX 78763-5080 15. Supplementary Notes Project conducted in cooperation with the Texas Department of Transportation and the U.S. Department of Transportation, Federal Highway Administration. 16. Abstract Liquid chemical products are marketed by a number of companies for stabilizing pavement base and subgrade soils. If effective, these products could be used as alternatives for treating sulfate-rich soils, which are susceptible to excessive heaving when treated with traditional, calcium-based stabilizers like lime, cement, and fly ash. However, the chemical composition, stabilizing mechanisms, and performance of these liquid products are not well understood. The primary objective of this study was to investigate and identify the mechanisms by which clay soils are modified or altered by these liquid chemical agents. -

ABSTRACT KOLELL, HANNAH JOY. Hydrophobic
ABSTRACT KOLELL, HANNAH JOY. Hydrophobic Disperse and Polymeric Dyes for the Coloration of UHMWPE Medical Sutures. (Under the direction of Dr. Harold S. Freeman). Ultra-high molecular weight polyethylene (UHMWPE) has extremely beneficial properties, including being high strength at a relatively low weight. However, its high crystallinity and very hydrophobic nature make coloration from a dyebath difficult. Attempts at surface modification of the polymer to increase dyeability often adversely affect the desired high-performance properties. UHMWPE has applications in the medical field, including as sutures, and for surgical purposes coloration at sufficient visibility levels is of high importance. Currently, there is one FDA certified dye, D&C Violet 2, that has the ability to dye UHMWPE sutures. Thus, having additional dyes is of interest as long as the resulting color is not red. To achieve this goal, the present study pertained to the modification of various FDA certified dyes to make them suitable for dyeing PE sutures. Specifically, dyes such as C.I. Solvent Yellow 18 and C.I. Solvent Green 3 (D&C Green 6) were modified with C-4 to C-6 alkyl groups and after structure confirmation through HPLC, UV-Vis, NMR, and HR-MS, the resulting 6 dyes were tested for their ability to dye UHMWPE fibers. The dyeing study showed visibly darker fibers from the modified dye structures in comparison to the D&C Violet 2 prototype. Overall the C-4 substituted dyes showed darker coloration of the fibers and in general showed lower dye release levels during extractions, making them the preferred choices for PE sutures. -

Additives for Polyolefins: Getting the Most out of Polypropylene
ADDITIVES FOR POLYOLEFINS PLASTICS DESIGN LIBRARY (PDL) PDL HANDBOOK SERIES Series Editor: Sina Ebnesajjad, PhD ([email protected]) President, FluoroConsultants Group, LLC Chadds Ford, PA, USA www.FluoroConsultants.com The PDL Handbook Series is aimed at a wide range of engineers and other professionals working in the plastics indus- try, and related sectors using plastics and adhesives. PDL is a series of data books, reference works, and practical guides covering plastics engineering, applications, proces- sing, and manufacturing, and applied aspects of polymer science, elastomers, and adhesives. Recent titles in the series Biopolymers: Processing and Products, Michael Niaounakis (ISBN: 9780323266987) Biopolymers: Reuse, Recycling, and Disposal, Michael Niaounakis (ISBN: 9781455731459) Carbon Nanotube Reinforced Composites, Marcio Loos (ISBN: 9781455731954) Extrusion, 2e, John Wagner and Eldridge Mount (ISBN: 9781437734812) Fluoroplastics, Volume 1, 2e, Sina Ebnesajjad (ISBN: 9781455731992) Handbook of Biopolymers and Biodegradable Plastics, Sina Ebnesajjad (ISBN: 9781455728343) Handbook of Molded Part Shrinkage and Warpage, Jerry Fischer (ISBN: 9781455725977) Handbook of Polymer Applications in Medicine and Medical Devices, Kayvon Modjarrad and Sina Ebnesajjad (ISBN: 9780323228053) Handbook of Thermoplastic Elastomers, Jiri G. Drobny (ISBN: 9780323221368) Handbook of Thermoset Plastics, 2e, Hanna Dodiuk and Sidney Goodman (ISBN: 9781455731077) High Performance Polymers, 2e, Johannes Karl Fink (ISBN: 9780323312226) Introduction -

Removal of Anionic Dye Congo Red from Aqueous Environment Using
www.nature.com/scientificreports OPEN Removal of anionic dye Congo red from aqueous environment using polyvinyl alcohol/sodium alginate/ ZSM‑5 zeolite membrane Sabarish Radoor1*, Jasila Karayil2, Jyotishkumar Parameswaranpillai1 & Suchart Siengchin1* In this study, a novel PVA/SA/ZSM‑5 zeolite membrane with good regeneration capacity was successfully prepared by solvent casting technique. The properties of the membranes were assessed by employing diferent characterization techniques such as X‑ray difraction (XRD), Fourier‑transform infrared spectroscopy (FT‑IR), scanning electron microscope (SEM), optical microscopy (OP), thermogravimetric analysis (TGA), contact angle and universal testing machine (UTM). XRD, TGA and UTM results revealed that the crystallinity and thermo‑mechanical performance of the membrane could be tuned with zeolite content. The successful incorporation of zeolite into the polymer matrix was confrmed by FT‑IR, SEM and OP analysis. The adsorption ability of the as‑prepared membrane was evaluated with a model anionic dye, Congo red. Adsorption studies show that the removal efciency of the membrane could be tuned by varying zeolite content, initial concentration of dye, contact time, pH and temperature. Maximum dye adsorption (5.33 mg/g) was observed for 2.5 wt% zeolite loaded membrane, at an initial dye concentration of 10 ppm, pH 3 and temperature 30 °C. The antibacterial efciency of the membrane against gram‑positive (Staphylococcus aureus) and gram‑negative bacteria (Escherichia coli) was also reported. The results show that membrane inhibits the growth of both gram‑positive and gram‑negative bacteria. The adsorption isotherm was studied using two models: Langmuir and Freundlich isotherm. The results show that the experimental data ftted well with Freundlich isotherm with a high correlation coefcient (R2 = 0.998). -

Kocetal Polyoxymethylene 1 1 Business Area
® Engineering Plastic KOCETAL POLYOXYMETHYLENE 1 1 BUSINESS AREA Seoul Ofce (Sales Division) Seoul Office (Sales Division) 1Seoul0th floor Ofce, Kolon (Sales Tow Division)er annex 1-22, Seoul Ofce (Sales Division) Byulyang-Dong, Gwacheon City, 10F, an annex to Kolon Tower 1-22, 10th floor, Kolon Tower annex 1-22, Seoul Ofce (Sales Division) ByulyGyunggi-Do,ang-Dong Korea, Gwacheon City, Byeolyang-dong, Gwacheon City, Gyunggi-Do, Korea Gyunggi-Do, Korea www.kolonplastics.com Headquarters and Plant www.kolonplastics.com Headquarters and Plant Headquarters, Factory and R&D Division Headquarters, Factory and R&D Division 1018, Eungmyeong-dong, Gimcheon-si, 101Headquarters,8, Eungmyung-dong Factory, Gimcand heon-CitR&D Divisiony, Gyeongsangbuk-do, Korea 101GyeongSangBukDo,8, Eungmyung-dong Korea, Gimcheon-City, GyTel:eongSangBukDo, +82-54-420-8491, K 8477orea TFel:ax: +82-54-420-8491, +82-54-420-8369 8477 Fax: +82-54-420-8369 Contact for further information If you want to inquire more detail information on product of KOLONPLASTIC,INC. follow the process written below. 1. Access the Internet hompage of KOLONPLASTIC, INC. www.kolonplastics.com/enghome 2. ‘SALES CONTACT’ category. 3. Then you can see contact number on E-mail, Tel or Fax according to regional groups. About Kolon Plastics Kolon Plastics-Growing with our customers as a POM Global Leader Kolon Plastics was established in March 1996 as a joint venture between Kolon Industries Inc. in Korea and Toray Industries Inc. in Japan. Production began in 1998 with capacity and sales of 25,000MT/year. After the 2nd factory line was completed, we produce 57,000MT of POM and 50,000MT of the other compouding materials a year. -

Crosslinked Poly(Vinyl Alcohol) Membranes
International Journal of ChemTech Research CODEN (USA): IJCRGG, ISSN: 0974-4290, ISSN(Online):2455-9555 Vol.10 No.3, pp 118-127, 2017 Synthesis and Physical-Chemical Characterization of DEA- Crosslinked Poly(Vinyl Alcohol) Membranes María T. Acevedo1*, Alejandra Tapia1, Álvaro Realpe1 1Department of Chemical Engineering, Research Group of Modeling of Particles and Processes, Engineering Faculty, Universidad de Cartagena, Colombia Abstract : In this research, crosslinked PVA was impregnated with 0, 15 and 20% of diethanolamine so as to study the effect of the alkanolamine on the physical-chemical properties which are connected to the CO2 separating process via membranes. Formaldehyde and a porous membrane of polisulfone were used as crosslinking agent and support, respectively. Membranes were characterized by FTIR, SEM-EDS, contact angle, water absorption and porosity measurements. The results suggest that although the water absorption in the bulk of polymer diminished when the percentage of diethnaolamine increased, the surface hydrophilicity and appearance of the membranes were not appreciably affected due to this modification. A favorable balance of properties such as water uptake, the porosity and the superficial hidrophilicity were achieved using the crosslinking and synthesis conditions proposed in this paper, considering the positive effect of those characteristics in the permeability/selectivity of CO2 through the membrane. Keyword : crosslinked PVA, diethanolamine, CO2 separating process, membranes, surface hydrophilicity. Introduction Pollution associated with emissions of carbon dioxide (CO2) is one of the preeminent environmental issues [1,2]. The high level of CO2 emission levels is arising from combustion of fossil fuel, accelerated increase in the energy consumption, population and industries, including cement and metal production, which have caused different kinds of devastating effects in particular the increase in global temperature with severe consequences on ecosystems, biodiversity and sea levels [3]. -
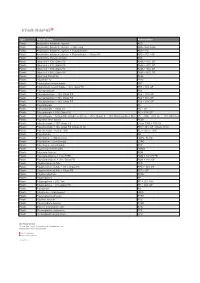
Type Material Name Abbreviation Plastic Acrylonitrile Butadiene
Type Material Name Abbreviation Plastic Acrylonitrile butadiene styrene ABS Plastic Acrylonitrile butadiene styrene - High-Temp ABS - high temp Plastic Acrylonitrile butadiene styrene + Polycarbonate ABS + PC Plastic Acrylonitrile butadiene styrene + Polycarbonate + Glass Fill ABS + PC + GF Plastic Acrylonitrile styrene acrylate ASA Plastic Nylon 6-6 + 10% Glass Fill PA66 + 10% GF Plastic Nylon 6-6 + 20% Glass Fill PA66 + 20% GF Plastic Nylon 6-6 + 30% Glass Fill PA66 + 30% GF Plastic Nylon 6-6 + 50% Glass Fill PA66 + 50% GF Plastic Nylon 6-6 Polyamide PA66 Plastic Polyamide 12 PA12 Plastic Polybutylene terephthalate PBT Plastic Polybutylene terephthalate + 30% Glass Fill PBT+ 30% GF Plastic Polycaprolactam PA6 Plastic Polycaprolactam + 20% Glass Fill PA6 + 20% GF Plastic Polycaprolactam + 30% Glass Fill PA6 + 30% GF Plastic Polycaprolactam + 50% Glass Fill PA6 + 50% GF Plastic Polycarbonate PC Plastic Polycarbonate + Glass Fill PC + GF Plastic Polycarbonate + 10% Glass Fill PC + 10% GF Plastic Polycarbonate + Acrylonitrile butadiene styrene + 20% Glass Fill + 10% Stainless Steel fiber PC + ABS + 20% GF + 10% SS Fiber Plastic Polyether ether ketone PEEK Plastic Polyetherimide + 30% Glass Fill Ultem 1000 + 30% GF Plastic Polyetherimide + 40% Glass Fill (Ultem 2410) PEI + 40% GF (Ultem 2410) Plastic Polyetherimide + Ultem 1000 PEI + Ultem 1000 Plastic Polyethylene PE Plastic Polyethylene - High-Density HDPE, PEHD Plastic Polyethylene - Low-Density LDPE Plastic Polyethylene terephthalate PET Plastic Polymethyl methacrylate PMMA Plastic Polyoxymethylene -

Process for Producing Polyoxymethylene-Polyurethane Type Alloy
~" ' MM II II II II II INI Ml I Ml II I II J European Patent Office _ _ _ _ © Publication number: 0 276 080 B1 Office_„. europeen des brevets © EUROPEAN PATENT SPECIFICATION © Date of publication of patent specification: 10.06.92 © Int. CI.5: C08G 18/66, C08L 59/00 © Application number: 88300257.8 @ Date of filing: 13.01.88 © Process for producing polyoxymethylene-polyurethane type alloy. © Priority: 23.01.87 JP 12294/87 © Proprietor: NIPPON POLYURETHANE INDUS- TRY CO. LTD. @ Date of publication of application: 2-8, Toranomon 1-chome 27.07.88 Bulletin 88/30 Mlnato-ku Tokyo(JP) © Publication of the grant of the patent: @ Inventor: Yano, Norlyoshl 10.06.92 Bulletin 92/24 1-13, Zushl 7-chome Zushl-shl Kanagawa-ken(JP) © Designated Contracting States: Inventor: Fujlta, Toshlhlko DE FR GB 422-14, Karlba-cho Hodogaya-ku Yokohama-shl Kanagawa-ken(JP) © References cited: EP-A- 0 167 369 US-A- 3 697 624 © Representative: West, Alan Harry et al R.G.C. Jenkins & Co. 26 Caxton Street "Polyurethanes Chemistry and Technology" London SW1H ORJ(GB) (Sauders,Frlsch) Krleger Pub. USA 1983, p. 401 "Textbook of Polymer Science" (Blllmeyer) Wiley Pub. USA 1971, p.237 "Properties of Polymers" (Krevelen), El- 00 sevier Pub. NL, 1976, p.495 00 CO CM O Note: Within nine months from the publication of the mention of the grant of the European patent, any person ^ may give notice to the European Patent Office of opposition to the European patent granted. Notice of opposition qj shall be filed in a written reasoned statement.