JETIR Research Journal
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

HŒ臬 A„簧綟糜恥sµ, Vw笑n® 22.12.2019 Š U拳 W
||Om Shri Manjunathaya Namah || Shri Kshethra Dhamasthala Rural Development Project B.C. Trust ® Head Office Dharmasthala HŒ¯å A„®ãtÁS®¢Sµ, vw¯ºN® 22.12.2019 Š®0u®± w®lµu® îµ±°ªæX¯Š®N®/ N®Zµ°‹ š®œ¯‡®±N®/w®S®u®± š®œ¯‡®±N® œ®±uµÛ‡®± wµ°Š® wµ°î®±N¯r‡®± ªRq® y®‹°£µ‡®± y®ªq¯ºý® D Nµ¡®w®ºruµ. Cu®Š®ªå 50 î®±q®±Ù 50 Oʺq® œµX®±Ï AºN® y®lµu®î®Š®w®±Ý (¬šµ¶g¬w®ªå r¢›Š®±î®ºqµ N®Zµ°‹/w®S®u®± š®œ¯‡®±N® œ®±uµÛSµ N®xÇ®Õ ïu¯ãœ®Áqµ y®u®ï î®±q®±Ù ®±š®±é 01.12.2019 NµÊ Aw®æ‡®±î¯S®±î®ºqµ 25 î®Ç®Á ï±°Š®u®ºqµ î®±q®±Ù îµ±ªæX¯Š®N® œ®±uµÛSµ N®xÇ®Õ Hš¬.Hš¬.HŒ¬.› /z.‡®±±.› ïu¯ãœ®Áqµ‡µ²ºvSµ 3 î®Ç®Áu® Nµ©š®u® Aw®±„Â®î® î®±q®±Ù ®±š®±é 01.12.2019 NµÊ Aw®æ‡®±î¯S®±î®ºqµ 30 î®Ç®Á ï±°Š®u®ºqµ ) î®±±ºvw® œ®ºq®u® š®ºu®ý®Áw®NµÊ B‡µ±Ê ¯l®Œ¯S®±î®¼u®±. š®ºu®ý®Áw®u® š®Ú¡® î®±q®±Ù vw¯ºN®î®w®±Ý y®äqµã°N®î¯T Hš¬.Hº.Hš¬ î®±²©N® ¯Ÿr x°l®Œ¯S®±î®¼u®±. œ¯cŠ¯u® HŒ¯å A„®ãtÁS®¢Sµ A†Ãw®ºu®wµS®¡®±. Written test Sl No Name Address Taluk District mark Exam Centre out off 100 11 th ward near police station 1 A Ashwini Hospete Bellary 33 Bellary kampli 2 Abbana Durugappa Nanyapura HB hally Bellary 53 Bellary 'Sri Devi Krupa ' B.S.N.L 2nd 3 Abha Shrutee stage, Near RTO, Satyamangala, Hassan Hassan 42 Hassan Hassan. -

Mmtc Unpaid Dividend 30092013
FNAME MNAME LNAME FHFNAME FHMNAME FHLNAME ADD COUNTRY STATE DISTRICT PIN FOLIO INVST AMOUNT DATE PARVEZ ANSARI NA NA Indra Nagar Bhel Jhansi U P INDIA Uttar Pradesh Jhansi 1201320000 Amount for Unclaimed 4.00 30-Oct-2016 282523 and Unpaid Dividend ZACHARIAH THOMAS NA NA Po Box 47257 Fahaheel KUWAIT NA 9NRI9999 1304140005 Amount for Unclaimed 12.00 30-Oct-2016 Fahaheel Kuwait 656021 and Unpaid Dividend ANKUR VILAS KULKARNI NA NA 25, River Dr South, Aptmt # UNITED NA 9NRI9999 1203440000 Amount for Unclaimed 16.00 30-Oct-2016 2511, Jersey City, Jersey Nj STATES OF 062447 and Unpaid Dividend Usa AMERICA ERAM HASHMI NA NA House No-3031 Kaziwara INDIA Delhi 110002 IN30011811 Amount for Unclaimed 4.00 30-Oct-2016 Darya Ganj New Delhi 249819 and Unpaid Dividend DURGA DEVI NA NA 451- Robin Cinema, Subzi INDIA Delhi 110007 1201911000 Amount for Unclaimed 4.00 30-Oct-2016 Mandi Delhi Delhi 017111 and Unpaid Dividend SOM DUTT DUEVEDI NA NA D-5/5, Ii Nd Floor Rana INDIA Delhi 110007 1203450000 Amount for Unclaimed 4.00 30-Oct-2016 Pratap Bagh New Delhi 443528 and Unpaid Dividend Delhi SUNIL GOYAL NA NA D 27 Cc Colony Opp Rana INDIA Delhi 110007 IN30114310 Amount for Unclaimed 4.00 30-Oct-2016 Pratap Bagh New Delhi 427000 and Unpaid Dividend AVM ENTERPRISES PVTLTD NA NA F 40, Okhla Ind Area Phase I INDIA Delhi 110020 IN30105510 Amount for Unclaimed 8.00 30-Oct-2016 New Delhi 266034 and Unpaid Dividend 30-Oct-2016 SUDARSHA H-80 Shivaji Park Punjabi IN30011811 Amount for Unclaimed GURPREET SINGH MEHTA N SINGH MEHTA Bagh New Delhi INDIA Delhi 110026 065484 -

District Census Handbook, Dharwad, Part XII-A, Series-11
CENSUS OF INDIA 1991 Series -11 KARNATAKA DISTRICT CENSUS HANDBOOK DHAR\VAD DISTRICT PART XII-A "IU.AGE ANHTOWN DIRELJORY SOBIIA NAMBISAN DH-ector of Census Operations, Kurnataka CONTENTS Page No. FOREWORD vii-viii PREFACE lX-X IMPORTANT STATISTICS ANALYTICAL NOTE Section-I - VHl&lge DiI'cctm'Y Explanatory Notc Alphabelical List of Villages - Bpdgi C.O.Blm:k Village Directory Statemcnt - B).tdgi C.D.Blud. Alphabetical List of Villages - Dhafwad CoD. Rlock Village Dircctory Statemcnt - Dharwad C.D.Block Alphahctical Liht of Villagch - (jadag C.O.BlI)(:k Village Directory Statement - Gadag CD.Block ()X- 105 Alphabetical Lihl of Villages - Hallgal C.D.Bhld: )1)1)- I 12 Village Directory Statement - Hangal CD.Block 11-1-1-11 Alphabetical Liht of Villages - Ha .... eri C.D.Block 145-147 Village Directory Stah:mcnl - 11a\L~ri C.D.Blod. 1-1X- J(,.1 Alphabetical Lihl of Vilbgl.: .... - } lirdcr lit' C.D.Block 1(,7-170 Village Directory Slal<.:m..:nl - I-lird. ..:rur C.D.BhK'1-.. 11'2-1:-;1) Alphabetical List of Villageh - lillbli C.D.BhlCk 1'J.1-194 ViI1age Directory Slat<.:ment - Hubli C.D.Block 1')(>-205 Alphabetical Liht of Villages - Kalg:h;tlgi C.D.Block 2()')- 21 I Village Dircctory Statcment - K4Ilghatgi CO.Block 212-225 Alphabclit'al List of Villages - K lInd;I~()1 CD.Block 22()-23() Village Directory Stat<.:ml'nt - Kundagul C.D.Block Alphabetical List of Villageh - MlInd,lrgi CD.B1o,:h Village Directory Statcl1ll:nt - l\lulllbrgi CO.Blnd P"g_L' l'< ll. Alphahctical Li~t 01" Villages - N :1I·g.und C [). -

Prl. District and Session Judge, Belagavi. Sri. Chandrashekhar Mrutyunjaya Joshi PRL. DISTRICT and SESSIONS JUDGE BELAGAVI Cause List Date: 12-10-2020
Prl. District and Session Judge, Belagavi. Sri. Chandrashekhar Mrutyunjaya Joshi PRL. DISTRICT AND SESSIONS JUDGE BELAGAVI Cause List Date: 12-10-2020 Sr. No. Case Number Timing/Next Date Party Name Advocate 11.00 AM-02.00 PM 1 SC 213/2019 The State of Karnataka R/by PP, Belagavi. PP (EVIDENCE) Vs Shravani Vinod Aralikatti R/o Plot No. 14 B J Gangai Alakapuri,Ramkrishnapurum,Kotapet,Haidrabad. 2 P.C.R. 7/2019 Manisha Ramesh Singh Thakur Age52yrs R/o M.M. SHAIKH (EVIDENCE) B.C No.1 Fort, Belagavi Vs Aashraya Infra Co-Ltd R/by Venkatesh Arjunrao Hishobkar R/o No.322 Roy road Tilakwadi Bgv 3 SC 327/2019 The State of Karnataka by Ramdurg PS PP (HBC) Vs Nagaraj Dyamanna Mali Age 39 yrs R/o Kankanawadi,Tq.Ramdurg,Dt.Belagavi. 4 SPL.C 1118/2019 The State of Karnataka R/by PP, Belagavi. PP (HBC) Vs Maruti Bhimappa Hulloli R/o Shindhikurabet, Tal.Gokak, Dt.Belagavi. 5 SC 21/2020 The State of Karnataka R/by PP, Belagavi. PP (Summans to accd) Vs Nikab @ Nikya Dastageerasab Peerajade Age. 36 years R/o Ashok Nagar, Belagavi. 6 Crl.Misc. 1532/2020 Krushnat @ Pintu S/o Rajaram Ghatage Age SRI. R. C. (OBJECTION) 32Yrs R/o Hanchinal K.S. Tq Nipani Dist INGALGI, Belagavi. Vs The State of karnataka Thorugh PSI Nippani Rural P.S. Nippani, R/by Learned P.P. Belagavi 7 Crl.Misc. 1586/2020 Arun S/o Channvaarayy Hiremath Age 25Yrs R/o Pattar (OBJECTION) Nirmal Nagar, Modaga, Belagavi. Shamsundar N Vs The State of Karnataka R/by P.P. -

The Karnataka Forest Manual
THE KARNATAKA FOREST MANUAL CONTENTS CHAPTER I CHAPTER I—CONSTITUTION OF RESERVED FORESTS 1 Procedure in respect of the constitution of a Reserved Forest 2 Selection and extent of Blocks 3 Areas to be included in the Forest 4 Areas to be excluded from Reservation 5 Boundaries of Blocks 6 Whole survey numbers to be taken 7 Collection of information in the Selection of Blocks 8 Preparation of maps 9 Preliminary demarcation 10 Draft Notification 11 Form of Notification 12 Forest Officer appointed to assist the Forest Settlement Officer 13 Form of proclamation 14 Inquiry by Forest Settlement Officer 15 Diary of Forest Settlement Officer 16 Privileges of Forest Officer attending the enquiry 17 Allowing time to Forest Officer to consult his higher authorities 18 Explaining the views of the Chief Conservator and the Conservator to the claimants and filling them for record 19 Procedure after admission of claims 20 Recording the rights of Forest tribes and important public rights of way 21 Form of notification 22 Permanent demarcation 23 Proclamation of the Notification under Section 18 in the regional language by the Deputy Commissioner 24 Final demarcation of boundaries 25 Defining of demarcated boundary by stone slabs, cairns etc. 26 Putting up of sign-boards 27 Forest Guards to be provided with Plans of their beats 28 Inspection and maintenance of Forest boundary lines and marks 29 Powers to re-notify boundaries of Reserved Forests 30 Declaration of forests no longer Reserved Forests 31 Acquisition of land 32 Forest Surveys and maps 33 Standardisation -

Bedkar Veedhi S.O Bengaluru KARNATAKA
pincode officename districtname statename 560001 Dr. Ambedkar Veedhi S.O Bengaluru KARNATAKA 560001 HighCourt S.O Bengaluru KARNATAKA 560001 Legislators Home S.O Bengaluru KARNATAKA 560001 Mahatma Gandhi Road S.O Bengaluru KARNATAKA 560001 Rajbhavan S.O (Bangalore) Bengaluru KARNATAKA 560001 Vidhana Soudha S.O Bengaluru KARNATAKA 560001 CMM Court Complex S.O Bengaluru KARNATAKA 560001 Vasanthanagar S.O Bengaluru KARNATAKA 560001 Bangalore G.P.O. Bengaluru KARNATAKA 560002 Bangalore Corporation Building S.O Bengaluru KARNATAKA 560002 Bangalore City S.O Bengaluru KARNATAKA 560003 Malleswaram S.O Bengaluru KARNATAKA 560003 Palace Guttahalli S.O Bengaluru KARNATAKA 560003 Swimming Pool Extn S.O Bengaluru KARNATAKA 560003 Vyalikaval Extn S.O Bengaluru KARNATAKA 560004 Gavipuram Extension S.O Bengaluru KARNATAKA 560004 Mavalli S.O Bengaluru KARNATAKA 560004 Pampamahakavi Road S.O Bengaluru KARNATAKA 560004 Basavanagudi H.O Bengaluru KARNATAKA 560004 Thyagarajnagar S.O Bengaluru KARNATAKA 560005 Fraser Town S.O Bengaluru KARNATAKA 560006 Training Command IAF S.O Bengaluru KARNATAKA 560006 J.C.Nagar S.O Bengaluru KARNATAKA 560007 Air Force Hospital S.O Bengaluru KARNATAKA 560007 Agram S.O Bengaluru KARNATAKA 560008 Hulsur Bazaar S.O Bengaluru KARNATAKA 560008 H.A.L II Stage H.O Bengaluru KARNATAKA 560009 Bangalore Dist Offices Bldg S.O Bengaluru KARNATAKA 560009 K. G. Road S.O Bengaluru KARNATAKA 560010 Industrial Estate S.O (Bangalore) Bengaluru KARNATAKA 560010 Rajajinagar IVth Block S.O Bengaluru KARNATAKA 560010 Rajajinagar H.O Bengaluru KARNATAKA -

Investor Detail.Xlsx
CINL51909DL1963GOI00 Compa MMTC LIMITED Date Of 30‐Sep‐2011 4033 ny AGM(DD‐ MON‐YYYY) Name Sum of unpaid and unclaimed dividend 24362.2 Sum of interest on unpaid and unclaimed 0 Sum of matured deposit 0 Sum of interest on matured deposit 0 Sum of matured debentures 0 Sum of interest on matured debentures 0 Sum of application money due for refund 0 Sum of interest on application money due for 0 First Name Middle Last Name Father/ Father/ Father/ Address Country State District PINCode Folio Number Investment Type Amount Proposed Name Husband Husban Husband of Securities Due(in Date of First d Last Rs.) transfer to Name Middle Name IEPF (DD‐ Name MON‐ YYYY) VIRENDRAB MAJMUDA 602,wilson Tower, Ghodod Road, Surat 000012010406 Amount for Unclaimed and 30‐Sep‐ SHIMUL HAI RNANASurat Gujarat INDIA Gujarat 395001 00017437 Unpaid Dividend 4.00 2016 Nehru Chowk Tah :‐ Waraseoni Dist :‐ Madhya 000012010601 Amount for Unclaimed and 30‐Sep‐ Vinay Kumar Surana NA NA Balaghat M.p. INDIA Pradesh 481331 00051312 Unpaid Dividend 4.00 2016 Singhwahini Chowk Chhindwara Madhya 000012010601 Amount for Unclaimed and 30‐Sep‐ LAXMI CHAND JAIN NA NA Madhyapradesh INDIA Pradesh 480447 00095261 Unpaid Dividend 4.00 2016 At Post Wani Near Sbi Wani Maharashtr 000012010601 Amount for Unclaimed and 30‐Sep‐ JYOTI DHANRAJ CHORDIYA NA NA Maharashtra INDIA a 445304 00095542 Unpaid Dividend 8.00 2016 CHANDRAK At Post Wani Near State Bank Road Maharashtr 000012010601 Amount for Unclaimed and 30‐Sep‐ UMAR PARASMAL CHORDIYA NA NA Wani Dist Yavatmal Maharashtra INDIA a 445304 00095557 Unpaid Dividend 8.00 2016 CHINTAMA Mahadeo Pura Ward No. -

S Name Address 2010.Xls
Name and Address of the BLO's Name of Assembly Constituency: 70-Kundgol Total No. of Parts in the AC: 163 Total No. of BLO's in the AC: 163 District: Dharwad Desgination Complete Address of Part No. Name of the BLO's Contact No. ( T / NT) the BLO's 1 2 3 4 5 Annapurna Kumbar NT At: Revadihal Ta: Hubli 1 Anganavadi Worker 9611381737 Sarojini Ballari NT At: Devaragudihal Ta:Hubli 2 Anganavadi Worker 9731617821 Kamalaxi Sahadevappa Arji NT At: Parasapur Ta: Hubli 3 Anganavadi Worker 9036409754 Anasuyya S Hosur NT At: Rayanal Ta: Hubli 4 Anganavadi Worker 9739570040 Manjunath M Madlingannavar NT At: Rayanal Ta: Hubli 5 9448786060 Gram Panchayat Clerk Channamma B Sirigeri NT At: Gangival Ta:Hubli 6 Anganavadi Worker 9902663988 Girijamma I Budnal NT At:Anchatageri Ta:Hubli 7 Anganavadi Worker PP-9972991196 Bhagyalaxami A Kambalagi NT At:Anchatageri Ta:Hubli Anganavadi Worker 8 9986170419 K. M. Shashikala Teacher At:A Timmasagar Ta:Hubli 9 Asst Master 9986971331 Kasturi Kamaladinni NT At:A Mavanur Ta:Hubli 10 Anganavadi Worker 9844413915 Kasturibai Sudi NT At:A Mavanur Ta:Hubli 11 Anganavadi Worker 9632426177 Shailaja V Kulakarni At:A Kotagondhunshi NT 12 Anganavadi Worker Ta:Hubli (0836) 2317160 Mahadevi Totad NT At: Kadapatti Ta: Kundgol 13 Anganavadi Worker 9916122906 S S Jadimath NT At: Allapur Ta: Kundgol 14 Anganavadi Worker 9986814192 V K Badiger Teacher At: Gudenakatti Ta:Kundgol 15 Asst Master 9008628870 Yashoda Nagappa Arkasali( At:Benakanahalli Badiger NT 16 Ta:Kunadgol 9845698944 Anganavadi Worker Indira D Topanagoudar NT At: Mullolli Ta:Kundgol 17 Anganavadi Worker 9972325606 Dyamavva Chalawadi At: Yarinarayanpur NT 18 Anganavadi Worker Ta:Kundgol 9481730761 Sharada M Shalavadi NT At:Yaraguppi Ta:Kudngol 19 Anganavadi Worker 08304-265316 Annapurna Vijayakumar Madivalar NT At: Yaraguppi Ta:Kudngol 20 Anganavadi Worker 9844202516 Page 1 of 8 Desgination Complete Address of Part No. -
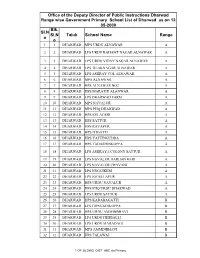
Sl.N O Blk. Sl.N O Taluk School Name Range Office Of
Office of the Deputy Director of Public Instructions Dharwad Range wise Government Primary School List of Dharwad as on 12- 05-2009 Blk. Sl.N Sl.N Taluk School Name Range o o 1 1 DHARWAD MPS URDU ALNAWAR A 2 2 DHARWAD LPS URDU RAHAMT NAGAR ALNAWAR A 3 3 DHARWAD LPS URDU VIDAY NAGAR ALNAWAR A 4 4 DHARWAD LPS TILAKNAGAR ALNAWAR A 5 5 DHARWAD LPS ASHRAY COL ALNAWAR A 6 6 DHARWAD MPS ALNAWAR A 7 7 DHARWAD HPS ALNAWAR NO2 A 8 8 DHARWAD HPS MARATHI ALANWAR A 9 9 DHARWAD LPS DHARWAD FARM A 10 10 DHARWAD MPS NAVALUR A 11 11 DHARWAD MPS PHQ DHARWAD A 12 12 DHARWAD HPS KELAGERI A 13 13 DHARWAD HPS SATTUR A 14 14 DHARWAD HPS RAYAPUR A 15 15 DHARWAD HPS ITIGATTI A 16 16 DHARWAD HPS YATTINGUDDA A 17 17 DHARWAD HPS TADASHINKOPPA A 18 18 DHARWAD LPS ASHRAYA COLONY SATTUR A 19 19 DHARWAD LPS NAVALUR HARIJAN KERI A 20 20 DHARWAD LPS NAVALUR CHAVANI A 21 21 DHARWAD LPS NUGGIKERI A 22 22 DHARWAD LPS JOGELLAPUR A 23 23 DHARWAD HPS URDU NAVALUR A 24 24 DHARWAD HPS PHQ URDU DHARWAD A 25 25 DHARWAD LPS URDU SATTUR A 26 26 DHARWAD HPS KADABAGATTI B 27 27 DHARWAD LPS GONGADIKOPPA B 28 28 DHARWAD HPS URDU AMMINBHAVI B 29 29 DHARWAD LPS URDU HEBBALLI B 30 30 DHARWAD LPS URDU MARADAGI B 31 31 DHARWAD MPS AMMINBHAVI B 32 32 DHARWAD HPS TALAWAI B 1 OF 28 DWD_DIST_ABC.xls Primary Range wise Government Primary School List of Dharwad as on 12- 05-2009 Blk. -

Process Documentation
Annex A Process documentation Annex A PROCESS DOCUMENTATION Dr Sangeetha Purushothaman and Simone Purohit Best Practices Foundation, Bangalore. _______________________________________________________________________ Chapter 1: Introduction....................................................................................................... 5 1.1. The Participatory Action Planning Project (PAPP)................................................ 5 1.2. Purpose of this annex............................................................................................... 5 1.3. Methodology............................................................................................................ 6 1.4. The Scope of this annex........................................................................................... 6 Chapter 2. Background ....................................................................................................... 8 2.1. The Hubli-Dharwad Peri-Urban Interface ............................................................... 8 2.2. Processes that led up to PAPP ................................................................................. 9 2.3. The PAPP Project .................................................................................................. 10 2.4. Partners in the PAPP Project.................................................................................. 12 Chapter 3. Setting the Tone: The Planning Workshop ..................................................... 15 3.1. Purpose of the Planning Meeting.......................................................................... -

S.No Name of Grma Panchayat Identified
SLBC KARNATAKA: GRAM PANCHAYAT WISE SERVICE AREA PLAN OF THE DISTRICT: DHARWAR Name of the District: Dharwar Name Of the Block: BR./BC/A Name of Name of all revenue Post Identified TM Population Of the villages forming the GP Village Code Office/S Name of Grma place/village of Block existing Sl.N the Village as S.No allotted Used in 2011 ubpost Panchayat 2000+populatio Name or o. per Census bank with Census Office n(2001 census) proposed 2001 Branch YES/NO with name 1 AMMINBHAVI Dharwar BR 1 AMMINBHAVI 602299 10889 YES 2 ARAVATAGI Dharwar BC 2 AMBOLI 602248 1344 NO Dharwar BR 3 ARAVATAGI 602247 944 YES Dharwar BC 4 KOGILAGERI 602238 1204 NO Dharwar BC 5 KUMBARKOPPA 602250 1694 NO 3 BELUR BELUR Dharwar VIJAYA KOTUR BC 1 BEILUR 602226 2280 NO Dharwar BC 2 HEGGARI 602231 650 NO Dharwar BC 3 NEERALKATTI 602220 1251 NO 4 BENACHI Dharwar BC 1 BALAGERI 602238 1204 NO Dharwar BC 2 BENAGHI 602250 1696 YES Dharwar BC 3 DORI 602249 1696 NO 5 DEVARHUBLI Dharwar BC 1 DEVARHUBLI 602265 1954 NO Dharwar BC 2 MALLURA 602279 42 NO Dharwar BC 3 MURAKATTI 602264 822 NO 6 GARAG Dharwar BC 1 AGASANHALLI 602213 149 NO Dharwar BR 2 GARAG 602214 9422 YES 7 HALLIGERI Dharwar BC 1 AMLIKOPPA 602267 849 NO Dharwar BC 2 HALLIGERI 602266 1287 YES Dharwar BC 3 HULTIKOTI 602258 1134 NO Dharwar BC 4 MAVINKOPPA 602259 461 NO Dharwar BC 5 RAGIKALLAPUR NO 8 HANGARKI Dharwar BC 1 DUBBANAMARADI 620212 500 NO HANGARKI Dharwar KVGB TADAKOD BC 2 HANGARKI 602206 2003 NO Dharwar BC 3 KM TADAKOD 602199 695 NO Dharwar BC 4 SHEDABALA 602207 89 YES 9 HAROBELAVADI HAROBELAVADI Dharwar -

District Census Handbook, Dharwad, Part XII-A & B, Series-30
CENSUS OF INDIA 2001 SERIES - 30 KARNATAKA PART XII - A & B : VILLAGE AND TOWN DIRECTORY & PRIMARY CENSUS ABSTRACT DISTRICT CENSUS HANDBOOK DHARWAD DIRECTORATE OF CENSUS OPERATIONS, KARNATAKA, BANGALORE Motif Unkal Tank near Hubli of Dharwad district Unkal Tank is located at a distance of 4 kms. from Unkal village is located at a distance of 4 kms., Hubli. Unkal Tank is one of the surviving tanks of from Hubli has a historical past. Unkal tank covers 250 Hubli City and will celeberate the centenary year dur acres with a catchment area of 4,651 Sq.Kms. A ing 2003. The then Municipal Authorities decided to number of migratory birds visits the tank. Due to rapid construct a tank near Unkal in 1886 in order to meet urbanization in the surrounding areas of the tank the the drinking water requirements of around 30,000 water supply has been stopped and declared unfit for people. Through the efforts of Great Statesman-Archi human consumption. Efforts to revive the past glory tect Sir.M.Vishweshwaraiah this project became reality for the tank is going on under National Tank Protection in 1903, it served as an important source of drinking Scheme water for the people of Hubli till the construction of Source : Deccan Herald Neerasagara and Malaprabha Reservoirs after Inde pendence. to 0 >. 0 ,., C\J ~ :0' @ • [EJ c .... ;:l ';::"" 0 >, ., ..Cl • I ::.:: 0 z ". u"" .I @ .3 I 00 ...." :a'" :5 ..s 'Ii '0 -' ~ <::: · • C Q) · E r.l 6 <::: · '- .... ;:0'-' Q) :> < I' '"0 0 '-' u '-' A N a< "" "' p:: >""a m co co Z < a "'" @ Z' ...