GEMIN4 Functions As a Coregulator of the Mineralocorticoid Receptor
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Nuclear Domains
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Cold Spring Harbor Laboratory Institutional Repository CELL SCIENCE AT A GLANCE 2891 Nuclear domains dynamic structures and, in addition, nuclear pore complex has been shown to rapid protein exchange occurs between have a remarkable substructure, in which David L. Spector many of the domains and the a basket extends into the nucleoplasm. Cold Spring Harbor Laboratory, One Bungtown nucleoplasm (Misteli, 2001). An The peripheral nuclear lamina lies Road, Cold Spring Harbor, NY 11724, USA extensive effort is currently underway by inside the nuclear envelope and is (e-mail: [email protected]) numerous laboratories to determine the composed of lamins A/C and B and is biological function(s) associated with thought to play a role in regulating Journal of Cell Science 114, 2891-2893 (2001) © The Company of Biologists Ltd each domain. The accompanying poster nuclear envelope structure and presents an overview of commonly anchoring interphase chromatin at the The mammalian cell nucleus is a observed nuclear domains. nuclear periphery. Internal patches of membrane-bound organelle that contains lamin protein are also present in the the machinery essential for gene The nucleus is bounded by a nuclear nucleoplasm (Moir et al., 2000). The expression. Although early studies envelope, a double-membrane structure, cartoon depicts much of the nuclear suggested that little organization exists of which the outer membrane is envelope/peripheral lamina as within this compartment, more contiguous with the rough endoplasmic transparent, so that internal structures contemporary studies have identified an reticulum and is often studded with can be more easily observed. -

Biogenesis of Nuclear Bodies
Downloaded from http://cshperspectives.cshlp.org/ on September 30, 2021 - Published by Cold Spring Harbor Laboratory Press Biogenesis of Nuclear Bodies Miroslav Dundr1 and Tom Misteli2 1Department of Cell Biology, Rosalind Franklin University of Medicine and Science, North Chicago, Ilinois 60064 2National Cancer Institute, National Institutes of Health, Bethesda, Maryland 20892 Correspondence: [email protected]; [email protected] The nucleus is unique amongst cellular organelles in that it contains a myriad of discrete suborganelles. These nuclear bodies are morphologically and molecularly distinct entities, and they host specific nuclear processes. Although the mode of biogenesis appears to differ widely between individual nuclear bodies, several common design principles are emerging, particularly, the ability of nuclear bodies to form de novo, a role of RNA as a struc- tural element and self-organization as a mode of formation. The controlled biogenesis of nuclear bodies is essential for faithful maintenance of nuclear architecture during the cell cycle and is an important part of cellular responses to intra- and extracellular events. he mammalian cell nucleus contains a mul- seems to act indirectly by regulating the local Ttitude of discrete suborganelles, referred to concentration of its components in the nucleo- as nuclear bodies or nuclear compartments plasm. (reviewed in Dundr and Misteli 2001; Spector In many ways, nuclear bodies are similar 2001; Lamond and Spector 2003; Handwerger to conventional cellular organelles in the cy- and Gall 2006; Zhao et al. 2009). These bodies toplasm. Like cytoplasmic organelles, they con- are an essential part of the nuclear landscape tain a specific set of resident proteins, which as they compartmentalize the nuclear space defines each structure molecularly. -

Towards an Understanding of Regulating Cajal Body Activity by Protein Modification
RNA BIOLOGY 2017, VOL. 14, NO. 6, 761–778 https://doi.org/10.1080/15476286.2016.1243649 REVIEW Towards an understanding of regulating Cajal body activity by protein modification Michael D. Hebert and Aaron R. Poole Department of Biochemistry, The University of Mississippi Medical Center, Jackson, MS, USA ABSTRACT ARTICLE HISTORY The biogenesis of small nuclear ribonucleoproteins (snRNPs), small Cajal body-specific RNPs (scaRNPs), Received 31 May 2016 small nucleolar RNPs (snoRNPs) and the telomerase RNP involves Cajal bodies (CBs). Although many Revised 30 August 2016 components enriched in the CB contain post-translational modifications (PTMs), little is known about how Accepted 27 September 2016 these modifications impact individual protein function within the CB and, in concert with other modified KEYWORDS factors, collectively regulate CB activity. Since all components of the CB also reside in other cellular Cajal body; coilin; locations, it is also important that we understand how PTMs affect the subcellular localization of CB phosphorylation; post- components. In this review, we explore the current knowledge of PTMs on the activity of proteins known translational modification; to enrich in CBs in an effort to highlight current progress as well as illuminate paths for future SMN; telomerase; WRAP53 investigation. Introduction functions of these proteins in the CB is diverse, but collectively There are different types of ribonucleoproteins (RNPs), which are thought to contribute to the RNP biogenesis mission of the are comprised of non-coding RNA and associated proteins. CB. Like other cellular processes, RNP biogenesis, and thus CB RNPs take part in fundamental cellular activities such as trans- activity, is regulated but an understanding of this regulation is lation and pre-mRNA splicing. -

THE ROLE of HISTONE LOCUS BODY (HLB) ASSEMBLY and the CELL CYCLE in HISTONE Mrna BIOSYNTHESIS
THE ROLE OF HISTONE LOCUS BODY (HLB) ASSEMBLY AND THE CELL CYCLE IN HISTONE mRNA BIOSYNTHESIS Esteban Alejandro Terzo A dissertation submitted in the faculty of the University of North Carolina at Chapel Hill in partial fulfillment of the requirements for the degree of Doctor of Philosophy in the Department of Biology. Chapel Hill 2015 Approved by: Robert J. Duronio William F. Marzluff Jeff Sekelsky Jean Cook Greg Matera © 2015 Esteban Alejandro Terzo ALL RIGHTS RESERVED ii ABSTRACT Esteban Alejandro Terzo: The role of histone locus body (HLB) assembly and the cell cycle in histone mRNA biosynthesis (Under the direction of Robert J. Duronio) The spatial compartmentalization of nuclear processes such as transcription, ribosome biogenesis, cellular response to stress, and histone mRNA biosynthesis into discrete territories is essential for precisely orchestrating diverse gene expression programs. The genome organizes structures called nuclear bodies (NBs) that concentrate factors (proteins, RNA, and Ribonucleoproteins) required for the efficient regulation of gene expression. The levels of the molecular components that constitute NBs fluctuate due to a continuous exchange with the nucleoplasm in response to diverse physiological inputs. Therefore, gaining insight into how NBs assemble and function is essential for understanding their contribution to genome function. We used the Drosophila histone locus body (HLB) as a model to ask how HLB assembly and function contribute to the replication-dependent histone gene expression. HLBs form at the histone locus and concentrate factors required for histone mRNA biosynthesis. In addition, CycE/Cdk2 is known to be required for cell cycle-dependent histone mRNA biosynthesis. Our laboratory previously demonstrated that the Multi Sex Combs (Mxc) protein is required for HLB assembly and efficient histone mRNA biosynthesis and is also phosphorylated by CycE/Cdk2. -

Snapshot: Cellular Bodies David L
SnapShot: Cellular Bodies David L. Spector Cold Spring Harbor Laboratory, Cold Spring Harbor, New York 11724, USA Number/ Typical Size Marker Body Name Description Image Cell and Shape Protein Involved in snRNP and snoRNP biogenesis and 0.1–2.0 µm; Cajal Body 0–6 Coilin posttranscriptional modification of newly assembled round spliceosomal snRNAs. 20S core Contains ubiquitin conjugates, the proteolytically active 0.2–1.2 µm; catalytic Clastosome 0–3 20S core and 19S regulatory complexes of the 26S irregular component of proteasome, and protein substrates of the proteasome. proteasome Contains several factors involved in 3′ cleavage of mRNAs. 0.2–1.0 µm; Cleavage Body 1–4 CstF 64 kDa ?20% contain newly synthesized RNA. Some cleavage round bodies localize adjacent to Cajal and PML bodies. Nuclear Contains proteins for pre-mRNA processing. Involved in Speckle or 0.8–1.8 µm; SC35, 25–50 the storage, assembly, and/or modification of pre-mRNA Interchromatin irregular SF2/ASF splicing factors. Granule Cluster Induced by heat shock response. Associates with Nuclear Stress 0.3–3.0 µm; satellite III repeats on human chromosome 9q12 and 2–10 HSF1 Body irregular other pericentromeric regions; recruits various RNA- binding proteins. Contains several transcription factors (Oct1/PTF) and 1.0–1.5 µm; OPT Domain 1–3 PTF RNA transcripts; predominant in late G1 cells. Often round Nuclear Bodies localizes close to nucleolus. 0.5 µm; Contains several RNA-binding proteins and nuclear- Paraspeckle 10–20 p54nrb, PSP1 round retained CTN-RNA. Cap on surface of nucleolus; found mainly in transformed Perinucleolar 0.3–1.0 µm; 1–4 hnRNPI (PTB) cells. -

Paraspeckles: Possible Nuclear Hubs by the RNA for the RNA
BioMol Concepts, Vol. 3 (2012), pp. 415–428 • Copyright © by Walter de Gruyter • Berlin • Boston. DOI 10.1515/bmc-2012-0017 Review Paraspeckles: possible nuclear hubs by the RNA for the RNA Tetsuro Hirose 1, * and Shinichi Nakagawa 2 Introduction 1 Biomedicinal Information Research Center , National Institute of Advanced Industrial Science and Technology, The eukaryotic cell nucleus is highly compartmentalized. 2-4-7 Aomi, Koutou 135-0064, Tokyo , Japan More than 10 membraneless subnuclear organelles have 2 RNA Biology Laboratory , RIKEN Advanced Research been identifi ed (1, 2) . These so-called nuclear bodies exist Institute, 2-1 Hirosawa, Wako 351-0198 , Japan in the interchromosomal space, where they are enriched in multiple nuclear regulatory factors, such as transcription and * Corresponding author RNA-processing factors. These factors are thought to serve e-mail: [email protected] as specialized hubs for various nuclear events, including transcriptional regulation and RNA processing (3, 4) . Some nuclear bodies serve as sites for the biogenesis of macromo- Abstract lecular machineries, such as ribosomes and spliceosomes. Multiple cancer cell types show striking alterations in their The mammalian cell nucleus is a highly compartmental- nuclear body organization, including changes in the numbers, ized system in which multiple subnuclear structures, called shapes and sizes of certain nuclear bodies (5) . The structural nuclear bodies, exist in the nucleoplasmic spaces. Some of complexity and dynamics of nuclear bodies have been impli- the nuclear bodies contain specifi c long non-coding RNAs cated in the regulation of complex gene expression pathways (ncRNAs) as their components, and may serve as sites for in mammalian cells. -

Integrins As New Governors of Nuclear Alterations?
cancers Review Inside the Cell: Integrins as New Governors of Nuclear Alterations? Elena Madrazo 1,2, Andrea Cordero Conde 2 and Javier Redondo-Muñoz 1,2,* 1 Section of Immuno-oncology, Instituto de Investigación Sanitaria Gregorio Marañón, 28007 Madrid, Spain; [email protected] 2 Department of Immunology, Hospital 12 de Octubre Health Research Institute (imas12), Complutense University School of Medicine, 28040 Madrid, Spain; [email protected] * Correspondence: [email protected]; Tel.: +34-913-941-642 Academic Editor: Helen M. Sheldrake Received: 16 May 2017; Accepted: 4 July 2017; Published: 6 July 2017 Abstract: Cancer cell migration is a complex process that requires coordinated structural changes and signals in multiple cellular compartments. The nucleus is the biggest and stiffest organelle of the cell and might alter its physical properties to allow cancer cell movement. Integrins are transmembrane receptors that mediate cell-cell and cell-extracellular matrix interactions, which regulate numerous intracellular signals and biological functions under physiological conditions. Moreover, integrins orchestrate changes in tumor cells and their microenvironment that lead to cancer growth, survival and invasiveness. Most of the research efforts have focused on targeting integrin-mediated adhesion and signaling. Recent exciting data suggest the crucial role of integrins in controlling internal cellular structures and nuclear alterations during cancer cell migration. Here we review the emerging role of integrins in nuclear biology. We highlight increasing evidence that integrins are critical for changes in multiple nuclear components, the positioning of the nucleus and its mechanical properties during cancer cell migration. Finally, we discuss how integrins are integral proteins linking the plasma membrane and the nucleus, and how they control cell migration to enable cancer invasion and infiltration. -

A Role for Protein Phosphatase PP1 in SMN Complex Formation And
A role for protein phosphatase PP1γ in SMN complex formation and subnuclear localization to Cajal bodies Benoît Renvoisé, Gwendoline Quérol, Eloi Rémi Verrier, Philippe Burlet, Suzie Lefebvre To cite this version: Benoît Renvoisé, Gwendoline Quérol, Eloi Rémi Verrier, Philippe Burlet, Suzie Lefebvre. A role for protein phosphatase PP1γ in SMN complex formation and subnuclear localization to Cajal bodies. Journal of Cell Science, Company of Biologists, 2012, 125 (12), pp.2862-2874. 10.1242/jcs.096255. hal-00776457 HAL Id: hal-00776457 https://hal.archives-ouvertes.fr/hal-00776457 Submitted on 20 Jan 2020 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. 2862 Research Article A role for protein phosphatase PP1c in SMN complex formation and subnuclear localization to Cajal bodies Benoıˆt Renvoise´ 1,*,`, Gwendoline Que´rol1,*, Eloi Re´mi Verrier1,§, Philippe Burlet2 and Suzie Lefebvre1," 1Laboratoire de Biologie Cellulaire des Membranes, Programme de Biologie Cellulaire, Institut Jacques-Monod, UMR 7592 CNRS, Universite´Paris Diderot, Sorbonne Paris Cite´, -

Nuclear Reorganization in Hippocampal Granule Cell Neurons from a Mouse Model of Down Syndrome: Changes in Chromatin Configuration, Nucleoli and Cajal Bodies
International Journal of Molecular Sciences Article Nuclear Reorganization in Hippocampal Granule Cell Neurons from a Mouse Model of Down Syndrome: Changes in Chromatin Configuration, Nucleoli and Cajal Bodies Alba Puente-Bedia 1, María T. Berciano 2, Olga Tapia 3 , Carmen Martínez-Cué 1 , Miguel Lafarga 4,*,† and Noemí Rueda 1,*,† 1 Department of Physiology and Pharmacology, Faculty of Medicine, University of Cantabria, 39011 Santander, Spain; [email protected] (A.P.-B.); [email protected] (C.M.-C.) 2 Department of Molecular Biology, “Red sobre Enfermedades Neurodegenerativas (CIBERNED)” and University of Cantabria-IDIVAL, 39011 Santander, Spain; [email protected] 3 Instituto de Investigación Sanitaria Valdecilla (IDIVAL), “Red sobre Enfermedades Neurodegenerativas (CIBERNED)” and Universidad Europea del Atlántico, 39011 Santander, Spain; [email protected] 4 Department of Anatomy and Cell Biology, “Red sobre Enfermedades Neurodegenerativas (CIBERNED)” and University of Cantabria-IDIVAL, 39011 Santander, Spain * Correspondence: [email protected] (M.L.); [email protected] (N.R.); Tel.: +34-942201966 (N.R.); Fax: +34-942201903 (N.R.) † These authors contributed equally to this work. Abstract: Down syndrome (DS) or trisomy of chromosome 21 (Hsa21) is characterized by impaired hippocampal-dependent learning and memory. These alterations are due to defective neurogenesis and to neuromorphological and functional anomalies of numerous neuronal populations, including Citation: Puente-Bedia, A.; Berciano, hippocampal granular -
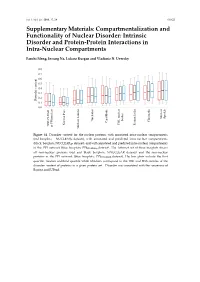
Intrinsic Disorder and Protein-Protein Interactions in Intra-Nuclear Compartments
Int. J. Mol. Sci. 2016, 17, 24 S1/S22 Supplementary Materials: Compartmentalization and Functionality of Nuclear Disorder: Intrinsic Disorder and Protein-Protein Interactions in Intra-Nuclear Compartments Fanchi Meng, Insung Na, Lukasz Kurgan and Vladimir N. Uversky 0.8 0.7 0.6 0.5 0.4 0.3 0.2 Disorder content 0.1 0.0 Nuclear Speckle bodies Nucleolus Chromatin Cajal Body Nuclear Pore PML nuclear Perinucleolar NNUCLEAR or PPInnuclear Nuclear Lamina Nuclear Figure S1. Disorder content for the nuclear proteins with annotated intra-nuclear compartments (red boxplots; NUCLEARa dataset), with annotated and predicted intra-nuclear compartments (black boxplots; NUCLEARap dataset), and with annotated and predicted intra-nuclear compartments in the PPI network (blue boxplots; PPINUCLEARap dataset). The leftmost set of three boxplots shows all non-nuclear proteins (red and black boxplots; NNUCLEAR dataset) and the non-nuclear proteins in the PPI network (blue boxplots; PPINNUCLEAR dataset). The box plots include the first quartile, median andthird quartile while whiskers correspond to the 10th and 90th centiles of the disorder content of proteins in a given protein set. Disorder was annotated with the consensus of Espritz and IUPred. Int. J. Mol. Sci. 2016, 17, 24 S2/S22 Table S1. Disorder content, fraction of disordered proteins, and abundance of disordered domains for the nuclear proteins in specific intra-nuclear compartments from the NUCLEARa and NUCLEARap datasets and for the non-nuclear proteins from the NNUCLEAR dataset. Disorder was annotated -

Exploring Mammalian Genome Within Phase-Separated Nuclear Bodies: Experimental Methods and Implications for Gene Expression
G C A T T A C G G C A T genes Review Exploring Mammalian Genome within Phase-Separated Nuclear Bodies: Experimental Methods and Implications for Gene Expression Annick Lesne 1,2,*, Marie-Odile Baudement 1,3 , Cosette Rebouissou 1 and Thierry Forné 1,* 1 IGMM, Univ. Montpellier, CNRS, F-34293 Montpellier, France; [email protected] (M.-O.B.); [email protected] (C.R.) 2 Sorbonne Université, CNRS, Laboratoire de Physique Théorique de la Matière Condensée, LPTMC, F-75252 Paris, France 3 Centre for Integrative Genetics (CIGENE), Faculty of Biosciences, Norwegian University of Life Sciences, 1430 Ås, Norway * Correspondence: [email protected] (A.L.); [email protected] (T.F.); Tel.: +33-434-359-682 (T.F.) Received: 6 November 2019; Accepted: 13 December 2019; Published: 17 December 2019 Abstract: The importance of genome organization at the supranucleosomal scale in the control of gene expression is increasingly recognized today. In mammals, Topologically Associating Domains (TADs) and the active/inactive chromosomal compartments are two of the main nuclear structures that contribute to this organization level. However, recent works reviewed here indicate that, at specific loci, chromatin interactions with nuclear bodies could also be crucial to regulate genome functions, in particular transcription. They moreover suggest that these nuclear bodies are membrane-less organelles dynamically self-assembled and disassembled through mechanisms of phase separation. We have recently developed a novel genome-wide experimental method, High-salt Recovered Sequences sequencing (HRS-seq), which allows the identification of chromatin regions associated with large ribonucleoprotein (RNP) complexes and nuclear bodies. -

Wholegenome Screening Identifies Proteins Localized to Distinct Nuclear Bodies
JCB: Tools Whole-genome screening identifies proteins localized to distinct nuclear bodies Ka-wing Fong,1 Yujing Li,2,3 Wenqi Wang,1 Wenbin Ma,2,3 Kunpeng Li,2 Robert Z. Qi,4 Dan Liu,5 Zhou Songyang,2,3,5 and Junjie Chen1 1Department of Experimental Radiation Oncology, The University of Texas MD Anderson Cancer Center, Houston, TX 77030 2Key Laboratory of Gene Engineering of Ministry of Education and 3State Key Laboratory of Biocontrol, School of Life Sciences, Sun Yat-sen University, Guangzhou 510275, China 4State Key Laboratory of Molecular Neuroscience, Division of Life Science, The Hong Kong University of Science and Technology, Hong Kong, China 5The Verna and Marrs McLean Department of Biochemistry and Molecular Biology, Baylor College of Medicine, Houston, TX 77030 he nucleus is a unique organelle that contains essen- 325 proteins localized to distinct nuclear bodies, including tial genetic materials in chromosome territories. The nucleoli (148), promyelocytic leukemia nuclear bodies (38), Tinterchromatin space is composed of nuclear sub- nuclear speckles (27), paraspeckles (24), Cajal bodies compartments, which are defined by several distinctive (17), Sam68 nuclear bodies (5), Polycomb bodies (2), nuclear bodies believed to be factories of DNA or RNA and uncharacterized nuclear bodies (64). Functional vali- processing and sites of transcriptional and/or posttranscrip- dation revealed several proteins potentially involved in tional regulation. In this paper, we performed a genome- the assembly of Cajal bodies and paraspeckles. Together, wide microscopy-based screening for proteins that form these data establish the first atlas of human proteins in nuclear foci and characterized their localizations using different nuclear bodies and provide key information for markers of known nuclear bodies.