Тип Mollusca Моллюски Пресноводные Моллюски: Примеры Viviparus Sp
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Title 2. List of Recorded Species Author(S)
Title 2. List of recorded species OHGAKI, SHUN-ICHI; KOMEMOTO, KEN-ICHI; Author(s) FUNAYAMA, NOBUTAKA Publications of the Seto Marine Biological Laboratory. Special Citation Publication Series (2011), 11: 4-14 Issue Date 2011 URL http://hdl.handle.net/2433/159498 Right Type Departmental Bulletin Paper Textversion publisher Kyoto University 2. List of recorded species Code no. Species name Japanese name Class Polyplacophora 多板綱 Family Ischnochitonidae ウスヒザラガイ科 1 Ischnochiton boninensis Bergenhayn ホソウスヒザラガイ 2 Ischnochiton comptus (Gould) ウスヒザラガイ Family Mopaliidae ヒゲヒザラガイ科 3 Placiphorella stimpsoni (Gould) ババガセ Family Chitonidae クサズリガイ科 4 Acanthopleura japonica (Lischke) ヒザラガイ 5 Acanthopleura loochooana (Broderip & リュウキュウヒザラガイ Sowerby) 6 Onithochiton hirasei Pilsbry ニシキヒザラガイ Family Acanthochitonidae ケハダヒザラガイ科 7 Acanthochitona achates (Gould) ヒメケハダヒザラガイ 8 Acanthochitona dissimilis Taki & Taki ビロウドヒザラガイ Family Cryptoplacidae ケムシヒザラガイ科 9 Cryptoplax japonica Pilsbry ケムシヒザラガイ Class Gastropoda 腹足綱 Family Patellidae ツタノハガイ科 10 Scutellastra flexuosa (Quoy & Gaimard) ツタノハ Family Nacellidae ヨメガカサガイ科 11 Cellana grata (Gould) ベッコウガサ 12 Cellana nigrolineata (Reeve) マツバガイ 13 Cellana toreuma (Reeve) ヨメガカサ Family Lottiidae ユキノカサガイ科 14 Patelloida pygmaea heroldi (Dunker) ヒメコザラ 4 15 Patelloida saccharina lanx (Reeve) ウノアシ 16 Lottia langfordi (Habe) キクコザラ 17 Lottia kogamogai Sasaki & Okutani コガモガイ 18 Lottia tenuisculpta Sasaki & Okutani コモレビコガモガイ 19 Lottia lindbergi Sasaki & Okutani オボロヅキコガモガイ 20 Nipponacmea fuscoviridis (Teramachi) クサイロアオガイ 21 Nipponacmea gloriosa (Habe) サクラアオガイ 22 Nipponacmea nigrans (Kira) クモリアオガイ 23 Nipponacmea schrenckii (Lischke) アオガイ Family Haliotidae ミミガイ科 24 Haliotis discus discus Reeve クロアワビ 25 Haliotis diversicolor aquatilis Reeve トコブシ 26 Haliotis varia Linnaeus イボアナゴ Family Fissurellidae スカシガイ科 27 Tugali decussata A. Adams シロスソカケガイ 28 Montfortula picta (Dunker) スソカケガイ 29 Macroschisma dilatatum (A. Adams) ヒラスカシガイ 30 Macroschisma sinense (A. Adams) スカシガイ Family Trochidae ニシキウズ科 31 Chlorostoma lischkei Tapparone-Canefri クボガイ 32 Chlorostoma turbinatum A. -

Morphology, 18S Rrna Gene Sequence and Life History of a New Polydora Species (Polychaeta: Spionidae) from Northeastern Japan
Vol. 18: 31–45, 2013 AQUATIC BIOLOGY Published online January 23 doi: 10.3354/ab00485 Aquat Biol Morphology, 18S rRNA gene sequence and life history of a new Polydora species (Polychaeta: Spionidae) from northeastern Japan Wataru Teramoto*, Waka Sato-Okoshi, Hirokazu Abe, Goh Nishitani, Yoshinari Endo Laboratory of Biological Oceanography, Graduate School of Agricultural Science, Tohoku University, Sendai 981-8555, Japan ABSTRACT: A new species of spionid polychaete, Polydora onagawaensis, is described from mol- lusk shells in Pacific waters of northeastern Japan. Its nuclear 18S rRNA gene sequence as well as its morphology, reproductive features, life history and infestation characteristics are reported. Polydora onagawaensis sp. nov. belongs to the Polydora ciliata/websteri group and has a moder- ate size and variable black pigmentation on the palps and body. Up to 115 worms were found bor- ing in a single scallop shell from suspended cultures in Onagawa Bay, with significantly higher numbers in the right than in the left valve. Females repeatedly deposited a string of egg capsules from around October to June (seawater temperature was below 15°C). The larvae developed inside the egg capsules for 2 wk (10°C, laboratory conditions), until the 3-chaetiger stage, before being released as planktonic larvae. The main spawning occurred in December, recruitment onto the shells increased after January, and most large worms disappeared between July and October. Thus, the estimated life span is around 1.5 yr after settlement. Details on biology and gene infor- mation not only contribute to distinguishing the species from other polydorids similar in morpho- logy, but also allow control of polydorid infestation in mollusk aquaculture. -

ABSTRACT Title of Dissertation: PATTERNS IN
ABSTRACT Title of Dissertation: PATTERNS IN DIVERSITY AND DISTRIBUTION OF BENTHIC MOLLUSCS ALONG A DEPTH GRADIENT IN THE BAHAMAS Michael Joseph Dowgiallo, Doctor of Philosophy, 2004 Dissertation directed by: Professor Marjorie L. Reaka-Kudla Department of Biology, UMCP Species richness and abundance of benthic bivalve and gastropod molluscs was determined over a depth gradient of 5 - 244 m at Lee Stocking Island, Bahamas by deploying replicate benthic collectors at five sites at 5 m, 14 m, 46 m, 153 m, and 244 m for six months beginning in December 1993. A total of 773 individual molluscs comprising at least 72 taxa were retrieved from the collectors. Analysis of the molluscan fauna that colonized the collectors showed overwhelmingly higher abundance and diversity at the 5 m, 14 m, and 46 m sites as compared to the deeper sites at 153 m and 244 m. Irradiance, temperature, and habitat heterogeneity all declined with depth, coincident with declines in the abundance and diversity of the molluscs. Herbivorous modes of feeding predominated (52%) and carnivorous modes of feeding were common (44%) over the range of depths studied at Lee Stocking Island, but mode of feeding did not change significantly over depth. One bivalve and one gastropod species showed a significant decline in body size with increasing depth. Analysis of data for 960 species of gastropod molluscs from the Western Atlantic Gastropod Database of the Academy of Natural Sciences (ANS) that have ranges including the Bahamas showed a positive correlation between body size of species of gastropods and their geographic ranges. There was also a positive correlation between depth range and the size of the geographic range. -

Bioenergetics of the Benthic Herbivorous Populations in a Rocky Intertidal Habitat
Bulletin of the Japanese Society of Scientific Fisheries 49(1), 33-40 (1983) Bioenergetics of the Benthic Herbivorous Populations in a Rocky Intertidal Habitat Mitsuhiro NAGATA* (Received May 31, 1982) Energy transformations through the population of rocky intertidal herbivores, Strongyl ocentrotus intermedius, Chlorostoma argyrostoma turbinatum and Omphalius rusticus, were ana lyzed from Oct. 1978 to Oct. 1979, using estimates of average weight and numbers of individuals in each size-group and observations on respiratory rates. The energy entering the population, which is the sum of the energy assimilated as food and the rate of immigrants, was 118.4 Kcal m-2 yr-1 for the Strongylocentrotus population, 12.6 Kcal m-2 yr-1 for the Chlorostoma popula tion, and 6.6 Kcal m-2 yr-1 for the Omphalius population. The amount of energy lost from the population, expressed as respiration, gametes ejected, predation, mortality and emigration, was estimated at 119.6, 13.4 and 6.5 Kcal m-2 yr-1 for the Strongylocentrotus, Chlorostoma and Omphalius populations, respectively. Since the rocky intertidal regions are strictly Hokkaido. The low-tide-platform, which meas exposed to changes in many environmental ured about 58.6•~104m2 in area, is about 3000 m factors, the rocky intertidal fauna is characterized along the shore-line and about 200m off-shore. largely by fluctuation in aspects of community The tidal excursion at this site is about 0.6 m in structure, including space utilization patterns, vertical distance, and 80% of the platform is composition, trophic structure, and body size exposed at low water. -

Florida Keys Species List
FKNMS Species List A B C D E F G H I J K L M N O P Q R S T 1 Marine and Terrestrial Species of the Florida Keys 2 Phylum Subphylum Class Subclass Order Suborder Infraorder Superfamily Family Scientific Name Common Name Notes 3 1 Porifera (Sponges) Demospongia Dictyoceratida Spongiidae Euryspongia rosea species from G.P. Schmahl, BNP survey 4 2 Fasciospongia cerebriformis species from G.P. Schmahl, BNP survey 5 3 Hippospongia gossypina Velvet sponge 6 4 Hippospongia lachne Sheepswool sponge 7 5 Oligoceras violacea Tortugas survey, Wheaton list 8 6 Spongia barbara Yellow sponge 9 7 Spongia graminea Glove sponge 10 8 Spongia obscura Grass sponge 11 9 Spongia sterea Wire sponge 12 10 Irciniidae Ircinia campana Vase sponge 13 11 Ircinia felix Stinker sponge 14 12 Ircinia cf. Ramosa species from G.P. Schmahl, BNP survey 15 13 Ircinia strobilina Black-ball sponge 16 14 Smenospongia aurea species from G.P. Schmahl, BNP survey, Tortugas survey, Wheaton list 17 15 Thorecta horridus recorded from Keys by Wiedenmayer 18 16 Dendroceratida Dysideidae Dysidea etheria species from G.P. Schmahl, BNP survey; Tortugas survey, Wheaton list 19 17 Dysidea fragilis species from G.P. Schmahl, BNP survey; Tortugas survey, Wheaton list 20 18 Dysidea janiae species from G.P. Schmahl, BNP survey; Tortugas survey, Wheaton list 21 19 Dysidea variabilis species from G.P. Schmahl, BNP survey 22 20 Verongida Druinellidae Pseudoceratina crassa Branching tube sponge 23 21 Aplysinidae Aplysina archeri species from G.P. Schmahl, BNP survey 24 22 Aplysina cauliformis Row pore rope sponge 25 23 Aplysina fistularis Yellow tube sponge 26 24 Aplysina lacunosa 27 25 Verongula rigida Pitted sponge 28 26 Darwinellidae Aplysilla sulfurea species from G.P. -
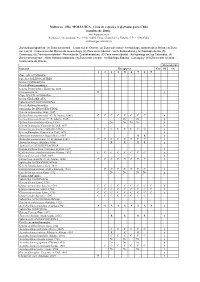
Moluscos - Filo MOLLUSCA
Moluscos - Filo MOLLUSCA. Lista de especies registradas para Cuba (octubre de 2006). José Espinosa Sáez Instituto de Oceanología, Ave 1ª No. 18406, Playa, Ciudad de La Habana, C.P. 11200, Cuba [email protected] Zonas biogeográficas: (1) Zona suroriental – Costa sur de Oriente, (2) Zona surcentral - Archipiélago Jardines de la Reina, (3) Zona sur central - Costa al sur del Macizo de Guamuhaya, (4) Zona suroccidental - Golfo de Batabanó y Archipiélago de los, (5) Canarreos, (6) Zona suroccidental - Península de Guanahacabibes, (7) Zona noroccidental - Archipiélago de Los Colorados, (8) Zona noroccidental - Norte Habana-Matanzas, (9) Zona norte-central - Archipiélago Sabana - Camagüey, (10) Zona norte-oriental - Costa norte de Oriente Abreviaturas Especies Bioegiones Cu Pl Oc 1 2 3 4 5 6 7 8 9 Clase APLACOPHORA Subclase SOLENOGASTRES Orden CAVIBELONIA Familia Proneomeniidae Género Proneomenia Hubrecht, 1880 Proneomenia sp . R x Clase POLYPLACOPHORA Orden NEOLORICATA Suborden ISCHNOCHITONINA Familia Ischnochitonidae Subfamilia ISCHNOCHITONINAE Género Ischnochiton Gray, 1847 Ischnochiton erythronotus (C. B. Adams, 1845) C C C C C C C C x Ischnochiton papillosus (C. B. Adams, 1845) Nc Nc x Ischnochiton striolatus (Gray, 1828) Nc Nc Nc Nc x Género Ischnoplax Carpenter in Dall, 1879 x Ischnoplax pectinatus (Sowerby, 1832) C C C C C C C C x Género Stenoplax Carpenter in Dall, 1879 x Stenoplax bahamensis Kaas y Belle, 1987 R R x Stenoplax purpurascens (C. B. Adams, 1845) C C C C C C C C x Stenoplax boogii (Haddon, 1886) R R R R x Subfamilia CALLISTOPLACINAE Género Callistochiton Carpenter in Dall, 1879 x Callistochiton shuttleworthianus Pilsbry, 1893 C C C C C C C C x Género Ceratozona Dall, 1882 x Ceratozona squalida (C. -

DNA Barcoding Reveal Patterns of Species Diversity Among
www.nature.com/scientificreports OPEN DNA barcoding reveal patterns of species diversity among northwestern Pacific molluscs Received: 04 April 2016 Shao’e Sun, Qi Li, Lingfeng Kong, Hong Yu, Xiaodong Zheng, Ruihai Yu, Lina Dai, Yan Sun, Accepted: 25 August 2016 Jun Chen, Jun Liu, Lehai Ni, Yanwei Feng, Zhenzhen Yu, Shanmei Zou & Jiping Lin Published: 19 September 2016 This study represents the first comprehensive molecular assessment of northwestern Pacific molluscs. In total, 2801 DNA barcodes belonging to 569 species from China, Japan and Korea were analyzed. An overlap between intra- and interspecific genetic distances was present in 71 species. We tested the efficacy of this library by simulating a sequence-based specimen identification scenario using Best Match (BM), Best Close Match (BCM) and All Species Barcode (ASB) criteria with three threshold values. BM approach returned 89.15% true identifications (95.27% when excluding singletons). The highest success rate of congruent identifications was obtained with BCM at 0.053 threshold. The analysis of our barcode library together with public data resulted in 582 Barcode Index Numbers (BINs), 72.2% of which was found to be concordantly with morphology-based identifications. The discrepancies were divided in two groups: sequences from different species clustered in a single BIN and conspecific sequences divided in one more BINs. In Neighbour-Joining phenogram, 2,320 (83.0%) queries fromed 355 (62.4%) species-specific barcode clusters allowing their successful identification. 33 species showed paraphyletic and haplotype sharing. 62 cases are represented by deeply diverged lineages. This study suggest an increased species diversity in this region, highlighting taxonomic revision and conservation strategy for the cryptic complexes. -

Pyramidelloides (Gastropoda Eulimidae) of the West Indies, with the Description Two New Species
BASTERIA, 54: 105-113, 1990 West Studies on Indian marine molluscs 18. Prosobranchia: in A review of the genus Pyramidelloides (Gastropoda Eulimidae) of the West Indies, with the description two new species M.J. Faber Zoological Museum, University of Amsterdam, P.O. Box 4766, 1009 AT Amsterdam, The Netherlands The in the West is reviewed. Four genusPyramidelloides (5.1.) Indies species are recognized, ofwhich P. and P. multicostatus. A trilirata De two are new to science, glaber syntype of Aclis shell-characters it is clear whether these Folin, 1873, is figured. Regarding only, not very four taxa belong to only one genus, or even to one family. West Indies. Key words: Gastropoda, Prosobranchia, Eulimidae, Pyramidelloides, taxonomy, INTRODUCTION 2 Waren (1984a), in his revision of the Pyramidelloides Nevill, 1885 proved that genus , this taxon (type-species Rissoa miranda A. Adams, 1861, by original designation) belongs to the Eulimidae, and not to the Rissoidae, to which it has often been relegated. He mentioned one species from the West Indies, viz. Aclis trilirata De Folin, 1872. From material in the Zoological Museum in Amsterdam it became clear that at least three other species of Pyramidelloides (5.1.) occur in the Caribbean. One, originally described as Cingulina? carinata Morch, 1876, has never been 3 lost from recorded since. The can be considered but the original description types , the species is recognizable, and not likely to be confused with any other West Indian mollusc taxon. It is figured here for the first time. The other two taxa are described as new. " Usticke (1971: 28) has listed a Pyramidelloides judithae Usticke, 1959". -

Proceedings of the United States National Museum
A MONOGRAPH OF WEST AMERICAN MELANELLID MOL- LUSKS. By Paul Bartsch, Curator, Division of Marine Invertebrates, United States National Museum. The present monograph completes the discussion of the West American Mollusks of the superfamily Pyramidelloideae, the Gym- noglossa, of Malacological Manuals. The superfamily consists of the families Pyramidellidae, which has been previously treated/ and the MelanelUdae, here considered. All the members of the superfamily are small mollusks, the largest attaining a size but little more than an inch in length. By far the greater number are elongate conic, but there are some which are quite rotund and others that range between these two extremes. In sculpture they vary from smooth to axially ribbed, to spirally striate or lirate, and combinations of these elements. Anatomically the members of this superfamily are differentiated from the other Proso- branchiate mollusks by the absence or extreme depauperation of the radula. The' members of the family PyramideUidae are readily distin- guished from those of the Melanellidae by the fact that the nepionic whorls are sinistral and tilted; the axis of the early whorls usually • The PyramiJellidae of the Marine Pliocene and Pleistocene Deposits of California, William H. Dall and Paul Bartsch, Mem. Cal. Acad. Sci., vol. 3, 1S03, pp. 269-285. SjTiopsis of the Genera, Subgenera, and section of the Family Pyramidellidae, William H. Dall and Paul Bartsch, Proc. Biol. Soc. Wash., vol. 17. 1904, pp. 1-16. Notes on Japanese, Indo- Pacific, and American Pyramidellidae, William H. Dall and Paul Bartsch, Proc. U. S. Nat. Mus., vol. 30, pp. 321-369, pis. 17-26, May 9, 1£06. -

Nuevas Observaciones Ecológicas Y Taxonómicas En Sabinella
Revista Mexicana de Biodiversidad 89 (2018): 123-133 Ecology New ecological and taxonomic remarks on Sabinella troglodytes and Nanobalcis worsfoldi (Gastropoda: Eulimidae) living on the “slate-pencil sea urchin” from the Mexican Caribbean region Nuevas observaciones ecológicas y taxonómicas en Sabinella troglodytes y Nanobalcis worsfoldi (Gastropoda: Eulimidae) que viven en el “erizo lápiz” de la región del Caribe mexicano Norma Emilia González-Vallejo a, b, *, Jesús Ángel de León-González b a Estructura y Función del Bentos, Depto. de Sistemática y Ecología Acuática, El Colegio de la Frontera Sur, Unidad Chetumal, Av. Centenario Km. 5.5, Chetumal, 77019 Quintana Roo, Mexico b Facultad de Ciencias Biológicas, Universidad Autónoma de Nuevo León, Cd. Universitaria, Pedro de Alba s/n, 66450 San Nicolás de los Garza, Nuevo León, México *Corresponding author: [email protected] (N.E. González-Vallejo) Received: 14 Marzo 2017; accepted: 07 August 2017 Abstract Eulimidae is one of the most diversified families among marine parasitic gastropods. They are usually reported associated with echinoderms, but for most described species the host is unknown, and few biological aspects of the symbiosis are known. As part of a larger study on eulimids, 300 sea urchins were collected in shallow water reef lagoons. Some were kept alive in the laboratory for 1 week and photographed and filmed under stereomicroscopes. Nanobalcis worsfoldi lives around and at the base of primary spines of the sea urchin Eucidaris tribuloides and is very abundant, whereas Sabinella troglodytes lives attached inside a gall that it builds from primary spines, and is uncommon. A complete characterization of the shells and morphology data for both eulimids are included. -

Larval Dispersal Dampens Population Fluctuation and Shapes the Interspecific Spatial Distribution Patterns of Rocky Title Intertidal Gastropods
Larval dispersal dampens population fluctuation and shapes the interspecific spatial distribution patterns of rocky Title intertidal gastropods Sahara, Ryosuke; Fukaya, Keiichi; Okuda, Takehiro; Hori, Masakazu; Yamamoto, Tomoko; Nakaoka, Masahiro; Noda, Author(s) Takashi Ecography, 38, 1-9 Citation https://doi.org/10.1111/ecog.01354 Issue Date 2015 Doc URL http://hdl.handle.net/2115/62537 Rights The definitive version is available at www.blackwell-synergy.com Type article (author version) File Information 150723ecography.pdf Instructions for use Hokkaido University Collection of Scholarly and Academic Papers : HUSCAP Larval dispersal dampens population fluctuation and shapes the interspecific spatial distribution patterns of rocky intertidal gastropods Ryosuke Sahara1, Keiichi Fukaya2, Takehiro Okuda3, Masakazu Hori4, Tomoko Yamamoto5, Masahiro Nakaoka6, and Takashi Noda1* 1Faculty of Environmental Science, Hokkaido University, N10W5, Kita-ku, Sapporo, Hokkaido 060-0810 Japan 2The Institute of Statistical Mathematics, 10-3 Midoricho, Tachikawa, Tokyo 190-8562 Japan 3National Research Institute of Far Seas Fisheries, Fisheries Research Agency, 2-12-4, Fukura, Kanazawa-ku, Yokohama 236-8648 Japan 4National Research Institute of Fisheries and Environment of Inland Sea, Fisheries Research Agency, Maruishi 2-17-5, Hatsukaichi, Hiroshima 739-0452 Japan 5Faculty of Fisheries, Kagoshima University, Shimoarata 4-50-20, Kagoshima, Kagoshima 890-0056 Japan 6Akkeshi Marine Station, Field Science Centre for the Northern Biosphere, Hokkaido University, Aikappu, Akkeshi, Hokkaido 088-1113 Japan *Corresponding author: Takashi NODA; email: [email protected] 1 Abstract Many marine benthic invertebrates pass through a planktonic larval stage whereas others spend their entire lifetimes in benthic habitats. Recent studies indicate that non-planktonic species show relatively greater fine-scale patchiness than do planktonic species, but the underlying mechanisms remain unknown. -

A Chronology of Middle Missouri Plains Village Sites
Smithsonian Institution Scholarly Press smithsonian contributions to botany • number 106 Smithsonian Institution Scholarly Press ConspectusA Chronology of the Benthic of MiddleMarine AlgaeMissouri of the Plains Gulf of California:Village Rhodophyta, Sites Phaeophyceae, and ChlorophytaBy Craig M. Johnson with contributions by StanleyJames A. N. Ahler, Norris, Herbert Luis Haas, E. and Aguilar-Rosas, Georges Bonani and Francisco F. Pedroche SERIES PUBLICATIONS OF THE SMITHSONIAN INSTITUTION Emphasis upon publication as a means of “diffusing knowledge” was expressed by the first Secretary of the Smithsonian. In his formal plan for the Institution, Joseph Henry outlined a program that included the following statement: “It is proposed to publish a series of reports, giving an account of the new discoveries in science, and of the changes made from year to year in all branches of knowledge.” This theme of basic research has been adhered to through the years by thousands of titles issued in series publications under the Smithsonian imprint, commencing with Smithsonian Contributions to Knowledge in 1848 and continuing with the following active series: Smithsonian Contributions to Anthropology Smithsonian Contributions to Botany Smithsonian Contributions to History and Technology Smithsonian Contributions to the Marine Sciences Smithsonian Contributions to Museum Conservation Smithsonian Contributions to Paleobiology Smithsonian Contributions to Zoology In these series, the Smithsonian Institution Scholarly Press (SISP) publishes small papers and