Evolution of Hypercarnivory: the Effect of Specialization on Morphological and Taxonomic Diversity
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
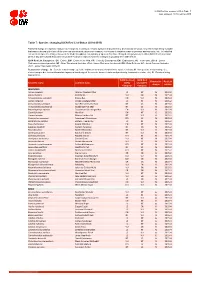
Table 7: Species Changing IUCN Red List Status (2014-2015)
IUCN Red List version 2015.4: Table 7 Last Updated: 19 November 2015 Table 7: Species changing IUCN Red List Status (2014-2015) Published listings of a species' status may change for a variety of reasons (genuine improvement or deterioration in status; new information being available that was not known at the time of the previous assessment; taxonomic changes; corrections to mistakes made in previous assessments, etc. To help Red List users interpret the changes between the Red List updates, a summary of species that have changed category between 2014 (IUCN Red List version 2014.3) and 2015 (IUCN Red List version 2015-4) and the reasons for these changes is provided in the table below. IUCN Red List Categories: EX - Extinct, EW - Extinct in the Wild, CR - Critically Endangered, EN - Endangered, VU - Vulnerable, LR/cd - Lower Risk/conservation dependent, NT - Near Threatened (includes LR/nt - Lower Risk/near threatened), DD - Data Deficient, LC - Least Concern (includes LR/lc - Lower Risk, least concern). Reasons for change: G - Genuine status change (genuine improvement or deterioration in the species' status); N - Non-genuine status change (i.e., status changes due to new information, improved knowledge of the criteria, incorrect data used previously, taxonomic revision, etc.); E - Previous listing was an Error. IUCN Red List IUCN Red Reason for Red List Scientific name Common name (2014) List (2015) change version Category Category MAMMALS Aonyx capensis African Clawless Otter LC NT N 2015-2 Ailurus fulgens Red Panda VU EN N 2015-4 -

Aspects of the Functional Morphology in the Cranial and Cervical Skeleton of the Sabre-Toothed Cat Paramachairodus Ogygia (Kaup, 1832) (Felidae
Blackwell Science, LtdOxford, UKZOJZoological Journal of the Linnean Society0024-4082The Lin- nean Society of London, 2005? 2005 1443 363377 Original Article FUNCTIONAL MORPHOLOGY OF P. OGYGIAM. J. SALESA ET AL. Zoological Journal of the Linnean Society, 2005, 144, 363–377. With 11 figures Aspects of the functional morphology in the cranial and cervical skeleton of the sabre-toothed cat Paramachairodus ogygia (Kaup, 1832) (Felidae, Machairodontinae) from the Late Miocene of Spain: Downloaded from https://academic.oup.com/zoolinnean/article-abstract/144/3/363/2627519 by guest on 18 May 2020 implications for the origins of the machairodont killing bite MANUEL J. SALESA1*, MAURICIO ANTÓN2, ALAN TURNER1 and JORGE MORALES2 1School of Biological & Earth Sciences, Byrom Street, Liverpool John Moores University, Liverpool, L3 3AF, UK 2Departamento de Palaeobiología, Museo Nacional de Ciencias Naturales-CSIC, José Gutiérrez Abascal, 2. 28006 Madrid, Spain Received January 2004; accepted for publication March 2005 The skull and cervical anatomy of the sabre-toothed felid Paramachairodus ogygia (Kaup, 1832) is described in this paper, with special attention paid to its functional morphology. Because of the scarcity of fossil remains, the anatomy of this felid has been very poorly known. However, the recently discovered Miocene carnivore trap of Batallones-1, near Madrid, Spain, has yielded almost complete skeletons of this animal, which is now one of the best known machairodontines. Consequently, the machairodont adaptations of this primitive sabre-toothed felid can be assessed for the first time. Some characters, such as the morphology of the mastoid area, reveal an intermediate state between that of felines and machairodontines, while others, such as the flattened upper canines and verticalized mandibular symphysis, show clear machairodont affinities. -

Carnivora from the Late Miocene Love Bone Bed of Florida
Bull. Fla. Mus. Nat. Hist. (2005) 45(4): 413-434 413 CARNIVORA FROM THE LATE MIOCENE LOVE BONE BED OF FLORIDA Jon A. Baskin1 Eleven genera and twelve species of Carnivora are known from the late Miocene Love Bone Bed Local Fauna, Alachua County, Florida. Taxa from there described in detail for the first time include the canid cf. Urocyon sp., the hemicyonine ursid cf. Plithocyon sp., and the mustelids Leptarctus webbi n. sp., Hoplictis sp., and ?Sthenictis near ?S. lacota. Postcrania of the nimravid Barbourofelis indicate that it had a subdigitigrade posture and most likely stalked and ambushed its prey in dense cover. The postcranial morphology of Nimravides (Felidae) is most similar to the jaguar, Panthera onca. The carnivorans strongly support a latest Clarendonian age assignment for the Love Bone Bed. Although the Love Bone Bed local fauna does show some evidence of endemism at the species level, it demonstrates that by the late Clarendonian, Florida had become part of the Clarendonian chronofauna of the midcontinent, in contrast to the higher endemism present in the early Miocene and in the later Miocene and Pliocene of Florida. Key Words: Carnivora; Miocene; Clarendonian; Florida; Love Bone Bed; Leptarctus webbi n. sp. INTRODUCTION can Museum of Natural History, New York; F:AM, Frick The Love Bone Bed Local Fauna, Alachua County, fossil mammal collection, part of the AMNH; UF, Florida Florida, has produced the largest and most diverse late Museum of Natural History, University of Florida. Miocene vertebrate fauna known from eastern North All measurements are in millimeters. The follow- America, including 43 species of mammals (Webb et al. -

Carnivora, Mammalia) in the Blancan of North America
THE PEARCE-SELLARDS Series NUMBER 19 THE GENUS DINOFELIS (CARNIVORA, MAMMALIA) IN THE BLANCAN OF NORTH AMERICA by BJORN KURTEN Submitted for publication May 30, 1972 TEXAS MEMORIAL MUSEUM / 24TH & TRINITY / AUSTIN, TEXAS W. W. NEWCOMB, JR., DIRECTOR The Genus Dinofelis (Carnivora, Mammalia) in the Blancan of North America Bjorn Kurten* INTRODUCTION The Blanco fauna was described by Meade (1945), who created the new on a felid species Panthera palaeoonca , based skull and associated mandible. Comparing the new species with members of the genera Panthera and Felis, he concluded that it showed close affinity to the jaguar. This has been tenta- tively accepted by later workers (Savage, 1960). The species forms the basis for the record of the genus Panthera in the Blancan of North America. A restudy of the material in 1971 led to the discovery in the collections of the Texas Memorial Museum at the University of Texas at Austin, of an additional, better preserved specimen (an upper canine) which clearly did not belong to Panthera or Felis but showed close affinity to the genus Dino- felis which has hitherto only been recorded from the Old World. Checking back on the type skull and mandible, the reference to Dinofelis could be fully substantiated. I wish to express my gratitude to Drs. John A. Wilson and Ernest L. Lundelius, the University of Texas at Austin, for the opportunity to study collections in their care. The abbreviation TMM is used to signify collections of the Texas Memorial Museum, the University of Texas at Austin. These were formerly in the collections of the Bureau of Economic Geology and in previous reports bore the prefix BEG. -

A NEW SABER-TOOTHED CAT from NEBRASKA Erwin H
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln Conservation and Survey Division Natural Resources, School of 1915 A NEW SABER-TOOTHED CAT FROM NEBRASKA Erwin H. Barbour Nebraska Geological Survey Harold J. Cook Nebraska Geological Survey Follow this and additional works at: http://digitalcommons.unl.edu/conservationsurvey Part of the Geology Commons, Geomorphology Commons, Hydrology Commons, Paleontology Commons, Sedimentology Commons, Soil Science Commons, and the Stratigraphy Commons Barbour, Erwin H. and Cook, Harold J., "A NEW SABER-TOOTHED CAT FROM NEBRASKA" (1915). Conservation and Survey Division. 649. http://digitalcommons.unl.edu/conservationsurvey/649 This Article is brought to you for free and open access by the Natural Resources, School of at DigitalCommons@University of Nebraska - Lincoln. It has been accepted for inclusion in Conservation and Survey Division by an authorized administrator of DigitalCommons@University of Nebraska - Lincoln. 36c NEBRASKA GEOLOGICAL SURVEY ERWIN HINCKLEY BARBOUR, State Geologist VOLUME 4 PART 17 A NEW SABER-TOOTHED CAT FROM NEBRASKA BY ERWIN H. BARBOUR AND HAROLD J. COOK GEOLOGICAL COLLECTIONS OF HON. CHARLES H. MORRILL 208 B A NEW SABER-TOOTHED CAT FROM NEBRASKA BY ERWIN H. BARBOUR AND HAROLD J, COOK During the field season of 1913, while exploring the Pliocene beds of Brown County, Mr. A. C. \Vhitford, a Fellow in the Department of Geology, University of Nebraska, discovered the mandible of a new mach.erodont cat. His work in this region was in the interest of the ~ebraska Geological Survey and the Morrill Geological Expeditions.1 The known fossil remains of the ancestral Felid.e fall into two nat ural lines of descent, as pointed out by Dr. -

Mammalia, Felidae, Canidae, and Mustelidae) from the Earliest Hemphillian Screw Bean Local Fauna, Big Bend National Park, Brewster County, Texas
Chapter 9 Carnivora (Mammalia, Felidae, Canidae, and Mustelidae) From the Earliest Hemphillian Screw Bean Local Fauna, Big Bend National Park, Brewster County, Texas MARGARET SKEELS STEVENS1 AND JAMES BOWIE STEVENS2 ABSTRACT The Screw Bean Local Fauna is the earliest Hemphillian fauna of the southwestern United States. The fossil remains occur in all parts of the informal Banta Shut-in formation, nowhere very fossiliferous. The formation is informally subdivided on the basis of stepwise ®ning and slowing deposition into Lower (least fossiliferous), Middle, and Red clay members, succeeded by the valley-®lling, Bench member (most fossiliferous). Identi®ed Carnivora include: cf. Pseudaelurus sp. and cf. Nimravides catocopis, medium and large extinct cats; Epicyon haydeni, large borophagine dog; Vulpes sp., small fox; cf. Eucyon sp., extinct primitive canine; Buisnictis chisoensis, n. sp., extinct skunk; and Martes sp., marten. B. chisoensis may be allied with Spilogale on the basis of mastoid specialization. Some of the Screw Bean taxa are late survivors of the Clarendonian Chronofauna, which extended through most or all of the early Hemphillian. The early early Hemphillian, late Miocene age attributed to the fauna is based on the Screw Bean assemblage postdating or- eodont and predating North American edentate occurrences, on lack of de®ning Hemphillian taxa, and on stage of evolution. INTRODUCTION southwestern North America, and ®ll a pa- leobiogeographic gap. In Trans-Pecos Texas NAMING AND IMPORTANCE OF THE SCREW and adjacent Chihuahua and Coahuila, Mex- BEAN LOCAL FAUNA: The name ``Screw Bean ico, they provide an age determination for Local Fauna,'' Banta Shut-in formation, postvolcanic (,18±20 Ma; Henry et al., Trans-Pecos Texas (®g. -

Title: Drimolen Crania Indicate Contemporaneity of Australopithecus, Paranthropus and Early Homo Erectus in S
Submitted Manuscript: Confidential Title: Drimolen crania indicate contemporaneity of Australopithecus, Paranthropus and early Homo erectus in S. Africa Authors: Andy I.R. Herries1,2*†, Jesse M. Martin1†, A.B. Leece1†, Justin W. Adams3,2†, Giovanni Boschian4,2†, Renaud Joannes-Boyau5,2, Tara R. Edwards1, Tom Mallett1, Jason Massey3,6, Ashleigh Murszewski1, Simon Neuebauer7, Robyn Pickering8.9, David Strait10,2, Brian J. Armstrong2, Stephanie Baker2, Matthew V. Caruana2, Tim Denham11, John Hellstrom12, Jacopo Moggi-Cecchi13, Simon Mokobane2, Paul Penzo-Kajewski1, Douglass S. Rovinsky3, Gary T. Schwartz14, Rhiannon C. Stammers1, Coen Wilson1, Jon Woodhead12, Colin Menter13 Affiliations: 1. Palaeoscience Labs, Dept. Archaeology and History, La Trobe University, Bundoora, 3086, VIC, Australia. 2. Palaeo-Research Institute, University of Johannesburg, Gauteng Province, South Africa. 3. Department of Anatomy and Developmental Biology, Biomedicine Discovery Institute, Monash University, VIC, Australia. 4. Department of Biology, University of Pisa, Italy 5. Geoarchaeology and Archaeometry Research Group (GARG), Southern Cross University, Military Rd, Lismore, 2480, NSW, Australia 6. Department of Integrative Biology and Physiology, University of Minnesota Medical School, USA 7. Department of Human Evolution, Max Planck Institute for Evolutionary Anthropology, Germany. 8. Department of Geological Sciences, University of Cape Town, Western Cape, South Africa 9. Human Evolution Research Institute, University of Cape Town, Western Cape, South Africa 10. Department of Anthropology, Washington University in St. Louis, St. Louis, USA 11. Geoarchaeology Research Group, School of Archaeology and Anthropology, Australian National University, Canberra, ACT, Australia 12. Earth Sciences, University of Melbourne, Australia 13. Department of Biology, University of Florence, Italy 14. Institute of Human Origins, School of Human Evolution and Social Change, Arizona State University, U.S.A. -

(Barbourofelinae, Nimravidae, Carnivora), from the Middle Miocene of China Suggests Barbourofelines Are Nimravids, Not Felids
UCLA UCLA Previously Published Works Title A new genus and species of sabretooth, Oriensmilus liupanensis (Barbourofelinae, Nimravidae, Carnivora), from the middle Miocene of China suggests barbourofelines are nimravids, not felids Permalink https://escholarship.org/uc/item/0g62362j Journal JOURNAL OF SYSTEMATIC PALAEONTOLOGY, 18(9) ISSN 1477-2019 Authors Wang, Xiaoming White, Stuart C Guan, Jian Publication Date 2020-05-02 DOI 10.1080/14772019.2019.1691066 Peer reviewed eScholarship.org Powered by the California Digital Library University of California Journal of Systematic Palaeontology ISSN: 1477-2019 (Print) 1478-0941 (Online) Journal homepage: https://www.tandfonline.com/loi/tjsp20 A new genus and species of sabretooth, Oriensmilus liupanensis (Barbourofelinae, Nimravidae, Carnivora), from the middle Miocene of China suggests barbourofelines are nimravids, not felids Xiaoming Wang, Stuart C. White & Jian Guan To cite this article: Xiaoming Wang, Stuart C. White & Jian Guan (2020): A new genus and species of sabretooth, Oriensmilusliupanensis (Barbourofelinae, Nimravidae, Carnivora), from the middle Miocene of China suggests barbourofelines are nimravids, not felids , Journal of Systematic Palaeontology, DOI: 10.1080/14772019.2019.1691066 To link to this article: https://doi.org/10.1080/14772019.2019.1691066 View supplementary material Published online: 08 Jan 2020. Submit your article to this journal View related articles View Crossmark data Full Terms & Conditions of access and use can be found at https://www.tandfonline.com/action/journalInformation?journalCode=tjsp20 Journal of Systematic Palaeontology, 2020 Vol. 0, No. 0, 1–21, http://dx.doi.org/10.1080/14772019.2019.1691066 A new genus and species of sabretooth, Oriensmilus liupanensis (Barbourofelinae, Nimravidae, Carnivora), from the middle Miocene of China suggests barbourofelines are nimravids, not felids a,bà c d Xiaoming Wang , Stuart C. -

O Ssakach Drapieżnych – Część 2 - Kotokształtne
PAN Muzeum Ziemi – O ssakach drapieżnych – część 2 - kotokształtne O ssakach drapieżnych - część 2 - kotokształtne W niniejszym artykule przyjrzymy się ewolucji i zróżnicowaniu zwierząt reprezentujących jedną z dwóch głównych gałęzi ewolucyjnych w obrębie drapieżnych (Carnivora). Na wczesnym etapie ewolucji, drapieżne podzieliły się (ryc. 1) na psokształtne (Caniformia) oraz kotokształtne (Feliformia). Paradoksalnie, w obydwu grupach występują (bądź występowały w przeszłości) formy, które bardziej przypominają psy, bądź bardziej przypominają koty. Ryc. 1. Uproszczone drzewo pokrewieństw ewolucyjnych współczesnych grup drapieżnych (Carnivora). Ryc. Michał Loba, na podstawie Nyakatura i Bininda-Emonds, 2012. Tym, co w rzeczywistości dzieli te dwie grupy na poziomie anatomicznym jest budowa komory ucha środkowego (bulla tympanica, łac.; ryc. 2). U drapieżnych komora ta jest budowa przede wszystkim przez dwie kości – tylną kaudalną kość entotympaniczną i kość ektotympaniczną. U kotokształtnych, w miejscu ich spotkania się ze sobą powstaje ciągła przegroda. Obydwie części komory kontaktują się ze sobą tylko za pośrednictwem małego okienka. U psokształtnych 1 PAN Muzeum Ziemi – O ssakach drapieżnych – część 2 - kotokształtne Ryc. 2. Widziane od spodu czaszki: A. baribala (Ursus americanus, Ursidae, Caniformia), B. żenety zwyczajnej (Genetta genetta, Viverridae, Feliformia). Strzałkami zaznaczono komorę ucha środkowego u niedźwiedzia i miejsce występowania przegrody w komorze żenety. Zdj. (A, B) Phil Myers, Animal Diversity Web (CC BY-NC-SA -

Paleoecological Comparison Between Late Miocene Localities of China and Greece Based on Hipparion Faunas
Paleoecological comparison between late Miocene localities of China and Greece based on Hipparion faunas Tao DENG Institute of Vertebrate Paleontology and Paleoanthropology, Chinese Academy of Sciences, Beijing 100044 (China) [email protected] Deng T. 2006. — Paleoecological comparison between late Miocene localities of China and Greece based on Hipparion faunas. Geodiversitas 28 (3) : 499-516. ABSTRACT Both China and Greece have abundant fossils of the late Miocene Hipparion fauna. Th e habitat of the Hipparion fauna in Greece was a sclerophyllous ever- green woodland. Th e Chinese late Miocene Hipparion fauna is represented respectively in the Guonigou fauna (MN 9), the Dashengou fauna (MN 10), and the Yangjiashan fauna (MN 11) from Linxia, Gansu, and the Baode fauna (MN 12) from Baode, Shanxi. According to the evidence from lithology, carbon isotopes, paleobotany, taxonomic framework, mammalian diversity and faunal similarity, the paleoenvironment of the Hipparion fauna in China was a subarid open steppe, which is diff erent from that of Greece. Th e red clay bearing the Hipparion fauna in China is windblown in origin, i.e. eolian deposits. Stable carbon isotopes from tooth enamel and paleosols indicate that C3 plants domi- nated the vegetation during the late Miocene in China. Pollens of xerophilous and sub-xerophilous grasses show a signal of steppe or dry grassland. Forest mammals, such as primates and chalicotheres, are absent or scarce, but grass- land mammals, such as horses and rhinoceroses, are abundant in the Chinese Hipparion fauna. Th e species richness of China and Greece exhibits a similar KEY WORDS trend with a clear increase from MN 9 to MN 12, but the two regions have Hipparion fauna, low similarities at the species level. -

University of Florida Thesis Or Dissertation Formatting
UNDERSTANDING CARNIVORAN ECOMORPHOLOGY THROUGH DEEP TIME, WITH A CASE STUDY DURING THE CAT-GAP OF FLORIDA By SHARON ELIZABETH HOLTE A DISSERTATION PRESENTED TO THE GRADUATE SCHOOL OF THE UNIVERSITY OF FLORIDA IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF DOCTOR OF PHILOSOPHY UNIVERSITY OF FLORIDA 2018 © 2018 Sharon Elizabeth Holte To Dr. Larry, thank you ACKNOWLEDGMENTS I would like to thank my family for encouraging me to pursue my interests. They have always believed in me and never doubted that I would reach my goals. I am eternally grateful to my mentors, Dr. Jim Mead and the late Dr. Larry Agenbroad, who have shaped me as a paleontologist and have provided me to the strength and knowledge to continue to grow as a scientist. I would like to thank my colleagues from the Florida Museum of Natural History who provided insight and open discussion on my research. In particular, I would like to thank Dr. Aldo Rincon for his help in researching procyonids. I am so grateful to Dr. Anne-Claire Fabre; without her understanding of R and knowledge of 3D morphometrics this project would have been an immense struggle. I would also to thank Rachel Short for the late-night work sessions and discussions. I am extremely grateful to my advisor Dr. David Steadman for his comments, feedback, and guidance through my time here at the University of Florida. I also thank my committee, Dr. Bruce MacFadden, Dr. Jon Bloch, Dr. Elizabeth Screaton, for their feedback and encouragement. I am grateful to the geosciences department at East Tennessee State University, the American Museum of Natural History, and the Museum of Comparative Zoology at Harvard for the loans of specimens. -

SMC 11 Gill 1.Pdf
SMITHSONIAN MISCELLANEOUS COLLECTIONS. 230 ARRANGEMENT FAMILIES OF MAMMALS. WITH ANALYTICAL TABLES. PREPARED FOR THE SMITHSONIAN INSTITUTION. BY THEODORE GILL, M.D., Ph.D. WASHINGTON: PUBLISHED BY THE SMITHSONIAN INSTITUTION. NOVEMBER, 1872. ADVERTISEMENT. The following list of families of Mammals, with analytical tables, has been prepared by Dr. Theodore Gill, at the request of the Smithsonian Institution, to serve as a basis for the arrangement of the collection of Mammals in the National Museum ; and as frequent applications for such a list have been received by the Institution, it has been thought advisable to publish it for more extended use. In provisionally adopting this system for the purpose mentioned, the Institution, in accordance with its custom, disclaims all responsibility for any of the hypothetical views upon which it may be based. JOSEPH HENRY, Secretary, S. I. Smithsonian Institution, Washington, October, 1872. (iii) CONTENTS. I. List of Families* (including references to synoptical tables) 1-27 Sub-Class (Eutheria) Placentalia s. Monodelpbia (1-121) 1, Super-Order Educabilia (1-73) Order 1. Primates (1-8) Sub-Order Anthropoidea (1-5) " Prosimiae (6-8) Order 2. Ferae (9-27) Sub-Order Fissipedia (9-24) . " Pinnipedia (25-27) Order 3. Ungulata (28-54) Sub-Order Artiodactyli (28-45) " Perissodactyli (46-54) Order 4. Toxodontia (55-56) . Order 5. Hyracoidea (57) Order 6. Proboscidea (58-59) Diverging (Educabilian) series. Order 7. Sirenia' (60-63) Order 8. Cete (64-73) . Sub-Order Zeuglodontia (64-65) " Denticete (66-71) . Mysticete (72-73) . Super-Order Ineducabilia (74-121) Order 9. Chiroptera (74-82) . Sub-Order Aniinalivora (74-81) " Frugivora (82) Order 10.