A New Occurrence of Gomesa Leinigii (Pabst) MW Chase & NH Williams
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Orchid of the Month for June, 2015 Oncidium Longipes by Bruce Adams
Orchid of the Month for June, 2015 Oncidium Longipes by Bruce Adams Figure 1: Oncidium longipes When I first fell in love with orchids, about forty years ago, Oncidium was my favorite genus. I loved the intricate flowers on long sprays, often with a wonderful fragrance. At that time, I worked as a volunteer in the orchid house at Planting Fields Arboretum. After repotting plants, I had the opportunity to take home back bulbs, and received pieces of Oncidium sphacelatum, O. flexuosum, and others that I can no longer remember. Every year they had an orchid auction, and for the extravagant price of five dollars, I purchased a multi-lead plant of O. ornithorhyncum. I became familiar with many of the various species, and at the time was a bit of an Oncidium expert. Forty years later, I’ve forgotten much, and with the recent changes in nomenclature maybe I wasn’t ever really an Oncidium expert, but rather a Trichocentrum, Gomesa, and Tolumnia expert! What hasn’t changed is my fondness for this vast genus (or group of genera). Plants can get quite large, such as Oncidium sphacelatum, which can easily can fill a twelve-inch pot, sending out three foot spikes with hundreds of flowers. But there are also miniatures like Oncidium harrisonianum, which can be contained in a three or four inch pot and sports short sprays of pretty little yellow flowers with brown spots. In fact, most Oncidium flowers are a variation of yellow and brown, although Oncidium ornithorhyncum produces pretty purple pink flowers, while Oncidium phalaenopsis and its relatives have beautiful white to red flowers, often spotted with pink. -

Phylogenetic Placement of the Enigmatic Orchid Genera Thaia and Tangtsinia: Evidence from Molecular and Morphological Characters
TAXON 61 (1) • February 2012: 45–54 Xiang & al. • Phylogenetic placement of Thaia and Tangtsinia Phylogenetic placement of the enigmatic orchid genera Thaia and Tangtsinia: Evidence from molecular and morphological characters Xiao-Guo Xiang,1 De-Zhu Li,2 Wei-Tao Jin,1 Hai-Lang Zhou,1 Jian-Wu Li3 & Xiao-Hua Jin1 1 Herbarium & State Key Laboratory of Systematic and Evolutionary Botany, Institute of Botany, Chinese Academy of Sciences, Beijing 100093, P.R. China 2 Key Laboratory of Biodiversity and Biogeography, Kunming Institute of Botany, Chinese Academy of Sciences, Kunming, Yunnan 650204, P.R. China 3 Xishuangbanna Tropical Botanical Garden, Chinese Academy of Sciences, Menglun Township, Mengla County, Yunnan province 666303, P.R. China Author for correspondence: Xiao-Hua Jin, [email protected] Abstract The phylogenetic position of two enigmatic Asian orchid genera, Thaia and Tangtsinia, were inferred from molecular data and morphological evidence. An analysis of combined plastid data (rbcL + matK + psaB) using Bayesian and parsimony methods revealed that Thaia is a sister group to the higher epidendroids, and tribe Neottieae is polyphyletic unless Thaia is removed. Morphological evidence, such as plicate leaves and corms, the structure of the gynostemium and the micromorphol- ogy of pollinia, also indicates that Thaia should be excluded from Neottieae. Thaieae, a new tribe, is therefore tentatively established. Using Bayesian and parsimony methods, analyses of combined plastid and nuclear datasets (rbcL, matK, psaB, trnL-F, ITS, Xdh) confirmed that the monotypic genus Tangtsinia was nested within and is synonymous with the genus Cepha- lanthera, in which an apical stigma has evolved independently at least twice. -

Histochemical and Phytochemical Analysis of Lamium Album Subsp
molecules Article Histochemical and Phytochemical Analysis of Lamium album subsp. album L. Corolla: Essential Oil, Triterpenes, and Iridoids Agata Konarska 1, Elzbieta˙ Weryszko-Chmielewska 1, Anna Matysik-Wo´zniak 2 , Aneta Sulborska 1,*, Beata Polak 3 , Marta Dmitruk 1,*, Krystyna Piotrowska-Weryszko 1, Beata Stefa ´nczyk 3 and Robert Rejdak 2 1 Department of Botany and Plant Physiology, University of Life Sciences, Akademicka 15, 20-950 Lublin, Poland; [email protected] (A.K.); [email protected] (E.W.-C.); [email protected] (K.P.-W.) 2 Department of General Ophthalmology, Medical University of Lublin, Chmielna 1, 20-079 Lublin, Poland; [email protected] (A.M.-W.); [email protected] (R.R.) 3 Department of Physical Chemistry, Medical University of Lublin, Chod´zki4A, 20-093 Lublin, Poland; [email protected] (B.P.); offi[email protected] (B.S.) * Correspondence: [email protected] (A.S.); [email protected] (M.D.); Tel.: +48-81-445-65-79 (A.S.); +48-81-445-68-13 (M.D.) Abstract: The aim of this study was to conduct a histochemical analysis to localize lipids, terpenes, essential oil, and iridoids in the trichomes of the L. album subsp. album corolla. Morphometric examinations of individual trichome types were performed. Light and scanning electron microscopy Citation: Konarska, A.; techniques were used to show the micromorphology and localization of lipophilic compounds and Weryszko-Chmielewska, E.; iridoids in secretory trichomes with the use of histochemical tests. Additionally, the content of Matysik-Wo´zniak,A.; Sulborska, A.; essential oil and its components were determined using gas chromatography-mass spectrometry Polak, B.; Dmitruk, M.; (GC-MS). -

Synoptic Overview of Exotic Acacia, Senegalia and Vachellia (Caesalpinioideae, Mimosoid Clade, Fabaceae) in Egypt
plants Article Synoptic Overview of Exotic Acacia, Senegalia and Vachellia (Caesalpinioideae, Mimosoid Clade, Fabaceae) in Egypt Rania A. Hassan * and Rim S. Hamdy Botany and Microbiology Department, Faculty of Science, Cairo University, Giza 12613, Egypt; [email protected] * Correspondence: [email protected] Abstract: For the first time, an updated checklist of Acacia, Senegalia and Vachellia species in Egypt is provided, focusing on the exotic species. Taking into consideration the retypification of genus Acacia ratified at the Melbourne International Botanical Congress (IBC, 2011), a process of reclassification has taken place worldwide in recent years. The review of Acacia and its segregates in Egypt became necessary in light of the available information cited in classical works during the last century. In Egypt, various taxa formerly placed in Acacia s.l., have been transferred to Acacia s.s., Acaciella, Senegalia, Parasenegalia and Vachellia. The present study is a contribution towards clarifying the nomenclatural status of all recorded species of Acacia and its segregate genera. This study recorded 144 taxa (125 species and 19 infraspecific taxa). Only 14 taxa (four species and 10 infraspecific taxa) are indigenous to Egypt (included now under Senegalia and Vachellia). The other 130 taxa had been introduced to Egypt during the last century. Out of the 130 taxa, 79 taxa have been recorded in literature. The focus of this study is the remaining 51 exotic taxa that have been traced as living species in Egyptian gardens or as herbarium specimens in Egyptian herbaria. The studied exotic taxa are accommodated under Acacia s.s. (24 taxa), Senegalia (14 taxa) and Vachellia (13 taxa). -

Genome Relationships in the Oncidium Alliance A
GENOME RELATIONSHIPS IN THE ONCIDIUM ALLIANCE A DISSERTATION SUBMITTED TO THE GRADUATE SCHOOL OF THE UNIVERSITY OF HAWAII IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF DOCTOR OF PHILOSOPHY IN HORTICULTURE MAY 1974 By Uthai Charanasri Dissertation Committee: Haruyuki Kamemoto, Chairman Richard W. Hartmann Peter P, Rotar Yoneo Sagawa William L. Theobald We certify that we have read this dissertation and that in our opinion it is satisfactory in scope and quality as a dissertation for the degree of Doctor of Philosophy in Horticulture. DISSERTATION COMMITTEE s f 1 { / r - e - Q TABLE OF CONTENTS Page LIST OF T A B L E S .............................................. iii LIST OF ILLUSTRATIONS...................................... iv INTRODUCTION ................................................ 1 REVIEW OF LITERATURE.................. 2 MATERIALS AND M E T H O D S ...................................... 7 RESULTS AND DISCUSSION ....................................... 51 Intraspecific Self- and Cross-Pollination Studies ........ Intrasectional Cross Compatibility within the Oncidium G e n u s ............................... 58 Intersectional and Intergeneric Hybridizations .......... 80 Chromosome Numbers ..................................... 115 K a r y o t y p e s ............................................ 137 Meiosis, Sporad Formation, and Fertility of Species Hybrids ............................. 146 Morphology of Species and Hybrids ..................... 163 General Discussion ................................... 170 SUMMARY -

Comparative Anatomy of the Floral Elaiophore in Representatives Of
Annals of Botany 112: 839–854, 2013 doi:10.1093/aob/mct149, available online at www.aob.oxfordjournals.org Comparative anatomy of the floral elaiophore in representatives of the newly re-circumscribed Gomesa and Oncidium clades (Orchidaceae: Oncidiinae) Małgorzata Stpiczyn´ska1, Kevin L. Davies2,*, Agata Pacek-Bieniek1 and Magdalena Kamin´ska1 1University of Life Sciences, Akademicka 15, 20-950 Lublin, Poland and 2School of Earth and Ocean Sciences, Cardiff University, Main Building, Park Place, Cardiff CF10 3AT, UK * For correspondence. E-mail [email protected] Downloaded from https://academic.oup.com/aob/article/112/5/839/139696 by guest on 29 September 2021 Received: 19 March 2013 Returned for revision: 8 April 2013 Accepted: 14 May 2013 Published electronically: 24 July 2013 † Background and Aims Recently, molecular approaches have been used to investigate the phylogeny of Oncidiinae. This has resulted in the transfer of taxa previously considered to be species of Oncidium Sw. into Gomesa R. Br. and the re-circumscription of both genera. In this study, the structure of the floral elaiophore (oil gland) is described and compared for Gomesa echinata (Barb. Rodr.) M.W. Chase & N.H. Williams, G. ranifera (Lindl.) M.W. Chase & N.H. Williams, Oncidium amazonicum (Schltr.) M.W. Chase & N.H. Williams and O. oxyceras (Ko¨niger & J.G. Weinm.) M.W. Chase & N.H. Williams in order to determine whether phylogenetic revision is supported by differences in its anatomy. † Methods The floral elaiophore structure was examined and compared at three developmental stages (closed bud, first day of anthesis and final stage of anthesis) for all four species using light microscopy, fluorescence microscopy, scanning electron microscopy, transmission electron microscopy and histochemistry. -

The Genus Systeloglossum
See discussions, stats, and author profiles for this publication at: https://www.researchgate.net/publication/256404679 The Genus Systeloglossum Article · January 1970 CITATION READS 1 60 2 authors: Robert Dressler Norris H Williams University of Costa Rica University of Florida 355 PUBLICATIONS 6,235 CITATIONS 131 PUBLICATIONS 4,002 CITATIONS SEE PROFILE SEE PROFILE Some of the authors of this publication are also working on these related projects: Sobralias of Costa Rica View project Determination of the new species of Sobralia found growing in the Turrialba Valley View project All content following this page was uploaded by Robert Dressler on 27 May 2014. The user has requested enhancement of the downloaded file. The Genus Systeloglossum ROBERT L. DRESSLER AND NORRIS H. WILLIAMS HE COMPLEX OF GENERA which make up the subtribe Oncidiinae are notable for their great diversity of flower structure. It is not surprising T that Schlechter grouped these genera into as many as ten different btribes. How can one imagine close relationships between Notylia and ~richoPilia, or Quekettia and Odontoglossum? In spite of their striking differences, we now group them all into the subtribe Oncidiinae, for two 'mportant reasons. First, crossing experiments, especially those of Mr. Moir, ~ave shown that the whole group is closely tied together by interfertility. We may not be able to cross Comparettia with Brassia, but we can cross both with Oncidium. The second reason for grouping them together is that even the morphological differences do not hold up very well on close inspection. Notylia may seem very different from Trichopilia, but Notylia and Macradenia are surely closely related, and Macradenia and Trichopilia subulata are perhaps more similar to each other than T . -

Texto Completo.Pdf
EVERALDO DA SILVA CRUZ TAXONOMY AND PHYLOGENY OF MYCORRHIZAL FUNGI ASSOCIATED WITH Gomesa recurva (ORCHIDACEAE) AND CHARACTERIZATION OF ENDOPHYTIC BACTERIA OF ORCHIDS Dissertação apresentada à Universidade Federal de Viçosa, como parte das exigências do Programa de Pós-Graduação em Microbiologia Agrícola, para obtenção do título de Magister Scientiae. Orientadora: Maria Catarina M. Kasuya Coorientadores: Meiriele da Silva Olinto Liparini Pereira VIÇOSA - MINAS GERAIS 2020 Ficha catalográfica elaborada pela Biblioteca Central da Universidade Federal de Viçosa - Campus Viçosa T Cruz, Everaldo da Silva, 1993- C957t Taxonomy and phylogeny of mycorrhizal fungi associated 2020 with Gomesa recurva (Orchidaceae) and characterization of endophytic bacteria of orchids / Everaldo da Silva Cruz. – Viçosa, MG, 2020. 47 f. : il. (algumas color.) ; 29 cm. Orientador: Maria Catarina Megumi Kasuya. Dissertação (mestrado) - Universidade Federal de Viçosa. Inclui bibliografia. 1. Filogenia. 2. Micorriza. 3. Fungos - Classificação. 4. Bactérias. 5. Hormônios vegetais. 6. Orquídea - Raízes. I. Universidade Federal de Viçosa. Departamento de Microbiologia. Programa de Pós-Graduação em Microbiologia Agrícola. II. Título. CDD 22. ed. 579.51785 A Deus, Aos meus pais, Aos meus irmãos, Aos meus amigos, Dedico. AGRADECIMENTOS À Universidade Federal de Viçosa pela estrutura e oportunidade da formação profissional, e ao Departamento de Microbiologia e ao Programa de Pós-Graduação em Microbiologia Agrícola pela oportunidade da realização da pós-graduação. Aos meus pais, Maria Aparecida e Antônio, por todo apoio, fé e amor. Exemplo de pessoas guerreiras, que moldaram minha educação e caráter, e que sem a luta deles eu não chegaria até aqui. Aos meus irmãos pela confiança e apoio durante todos esses anos de Universidade. -

Annotated Checklist of Vascular Flora, Cedar Breaks National
National Park Service U.S. Department of the Interior Natural Resource Program Center Annotated Checklist of Vascular Flora Cedar Breaks National Monument Natural Resource Technical Report NPS/NCPN/NRTR—2009/173 ON THE COVER Peterson’s campion (Silene petersonii), Cedar Breaks National Monument, Utah. Photograph by Walter Fertig. Annotated Checklist of Vascular Flora Cedar Breaks National Monument Natural Resource Technical Report NPS/NCPN/NRTR—2009/173 Author Walter Fertig Moenave Botanical Consulting 1117 W. Grand Canyon Dr. Kanab, UT 84741 Editing and Design Alice Wondrak Biel Northern Colorado Plateau Network P.O. Box 848 Moab, UT 84532 February 2009 U.S. Department of the Interior National Park Service Natural Resource Program Center Fort Collins, Colorado The Natural Resource Publication series addresses natural resource topics that are of interest and applicability to a broad readership in the National Park Service and to others in the management of natural resources, including the scientifi c community, the public, and the NPS conservation and environmental constituencies. Manuscripts are peer-reviewed to ensure that the information is scientifi cally credible, technically accurate, appropriately written for the intended audience, and is designed and published in a professional manner. The Natural Resource Technical Report series is used to disseminate the peer-reviewed results of scientifi c studies in the physical, biological, and social sciences for both the advancement of science and the achievement of the National Park Service’s mission. The reports provide contributors with a forum for displaying comprehensive data that are often deleted from journals because of page limitations. Current examples of such reports include the results of research that addresses natural resource management issues; natural resource inventory and monitoring activities; resource assessment reports; scientifi c literature reviews; and peer- reviewed proceedings of technical workshops, conferences, or symposia. -

2020-05 KOS Monthly Bulletin May 2020
THE MONTHLY BULLETIN OF THE KU-RING-GAI ORCHID SOCIETY INC. (Established in 1947) A.B.N. 92 531 295 125 May 2020 Volume 61 No. 5 Annual Membership : $15 single, $18 family . President : Dennys Angove 043 88 77 689 Committee Jessie Koh (Membership Secretary / Social Events) Secretary : Jenny Richardson (Culture Classes) Committee Herb Schoch (Liaison) Treasurer : Lina Huang Committee : Pauline Onslow (Member Support) Senior Vice President : tba Committee : Trevor Onslow (Guest Speakers) Junior Vice President : tba Committee : Chris Wilson (Library and Reference Sources) Editor (Hon volunteer) Jim Brydie Committee : Lee Payne (Sponsorship) Society mail to - PO box 1501 Lane Cove, NSW, 1595 Email – [email protected] web site (active link) : http:/kuringaiorchidsociety.org.au Next Meeting : * * * May Meeting CANCELLED With the present Corona virus situation, there will be no May meeting. The situation is constantly under review as to when we might resume. You will be advised immediately if there is a change. Wow, what a virtual benching – Wow, and Wow again. When virtual benching was first proposed I thought it might take members a little while to get on board with the idea. But no, there was terrific participation right from the start and a magical 6 page array of delicious, very professionally presented orchids, was created by Jenny. It included Cattleyas of all kinds and colours, Dendrobiums, Oncidiinae hybrids and rare species. It was just amazing. 14 different members contributed and if you count husbands and wives as separate it would be even more. The Fulchers provided a whole page of photos of orchids in flower from their collection, and even added a little info on each. -

Macradenia Grandiflora (Cymbidieae; Epidendroideae; Orchidaceae), a New Species from Southeastern Brazil
Phytotaxa 204 (2): 172–175 ISSN 1179-3155 (print edition) www.mapress.com/phytotaxa/ PHYTOTAXA Copyright © 2015 Magnolia Press Article ISSN 1179-3163 (online edition) http://dx.doi.org/10.11646/phytotaxa.204.2.9 Macradenia grandiflora (Cymbidieae; Epidendroideae; Orchidaceae), a new species from southeastern Brazil ANA KELLY KOCH1,2, ANNA LUIZA ILKIU-BORGES2 & FÁBIO DE BARROS1 1Instituto de Botânica de São Paulo, Núcleo de Pesquisa Orquidário do Estado. Av. Miguel Estéfano, 3687, Água Funda, São Paulo, São Paulo, Brazil; e-mail: [email protected] 2Museu Paraense Emilio Goeldi, Coordenação de Botânica. Av. Perimetral, 1901, Montese, Belém, São Paulo, Brazil. Abstract Macradenia grandiflora a new species from southeastern Brazil is described and illustrated. It is related to M. lutescens, but differs by the number of lip calli, the size of sepals, petals and lip, the number of flowers per inflorescence, the cuspidate apex of sepals and petals, and the irregularly lacerate margin of the clinandrium. Key words: Macradenia, Oncidiinae, southeastern Brazil Introduction Macradenia Brown (1822: 612) is a small Neotropical genus of the subtribe Oncidiinae, which is closely related to eight genera of the “twig epiphyte clade” (Chase et al. 2009). Currently, the genus is composed by 12 species, ranging from Mexico and south Florida, throughout Central America, West Indies, to Bolivia, Brazil and Peru, growing in places with high humidity from sea level up to 300 m above sea level (Pupulin & Ossenbach 2002, Chase et al. 2009). In Brazil, Macradenia is represented by 10 species distributed in 13 states (Barros et al. 2015). The genus was described by Robert Brown in 1822, however it was never reviewed. -
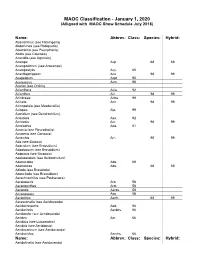
MAOC Classification - January 1, 2020 (Alligned with MAOC Show Schedule July 2018)
MAOC Classification - January 1, 2020 (Alligned with MAOC Show Schedule July 2018) Name: Abbrev.: Class: Species: Hybrid: Abaxianthus (see Flickingeria) Abdominea (see Robiquetia) Aberrantia (see Pleurothallis) Abola (see Caucaea) Acacallis (see Aganisia) Acampe Acp. 68 69 Acampodorum (see Aracampe) Acampostylis Acy. 65 Acanthephippium Aca. 98 99 Acapetalum Acpt. 95 Acemannia Acm. 98 Aceras (see Orchis) Acianthera Acia. 92 Acianthus Aci. 98 99 Acinbreea Acba. 99 Acineta Acn. 98 99 Acinopetala (see Masdevallia) Aciopea Aip. 99 Acoridium (see Dendrochilum) Acostaea Asa. 92 Acriopsis Acr. 98 99 Acrolophia Apa. 81 Acronia (see Pleurothallis) Acropera (see Gongora) Acrorchis Arr. 98 99 Ada (see Brassia) Adacidium (see Brassidium) Adaglossum (see Brassidium) Adapasia (see Brapasia) Adelopetalum (see Bulbophyllum) Adenocalpa Adp. 69 Adenoncos Ade. 68 69 Adioda (see Brassioda) Adonclioda (see Brassidium) Aerachnochilus (see Paulsenara) Aerangaeris Arg. 58 Aeranganthes Argt. 58 Aerangis Aergs. 58 Aerangopsis Ags. 58 Aeranthes Aerth. 68 69 Aerasconetia (see Aeridovanda) Aeridachnanthe Aed. 66 Aeridachnis Aerdns. 66 Aeridanthe (see Aeridovanda) Aerides Aer. 66 Aeridisia (see Luisaerides) Aeriditis (see Aeridopsis) Aeridocentrum (see Aeridovanda) Aeridochilus Aerchs. 66 Name: Abbrev.: Class: Species: Hybrid: Aeridofinetia (see Aeridovanda) Aeridoglossum (see Renades) Aeridoglottis Aegts. 66 Aeridopsis Aerps. 66 Aeridopsisanthe (see Maccoyara) Aeridostachya (see Eria) Aeridovanda Aerdv. 69 Aeridovanisia Aervsa. 66 Aeridsonia (see Aeridovanda) Aeristomanda Atom. 66 Aeroeonia Aoe. 58 Aetheorhyncha 98 99 Agananthes Agths. 95 Aganax (see Pabanisia) Aganella All. 78 Aganisia Agn. 95 Aganopeste Agt. 93 Aganulophia Agp. 81 Agasepalum Agsp. 95 Agrostophyllum Agr. 98 99 Aitkenara Aitk. 95 Alamania Al. 16 25 Alangreatwoodara (see Propabstopetalum) Alantuckerara Atc. 95 Alaticaulia (see Masdevallia) Alatiglossum (see Gomesa) Alcockara (see Ledienara) Alexanderara (see Brassidium) Aliceara Alcra.