Stock Assessment and Spatial Distribution of the Smooth Clam
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Lioconcha Castrensis Species Group (Bivalvia : Veneridae), with the Description of Two New Species
Molluscan Research 30(3): 117–124 ISSN 1323-5818 http://www.mapress.com/mr/ Magnolia Press The Lioconcha castrensis species group (Bivalvia : Veneridae), with the description of two new species SANCIA E.T. VAN DER MEIJ1, ROBERT G. MOOLENBEEK2 & HENK DEKKER2 1 Netherlands Centre for Biodiversity Naturalis (department of Marine Zoology), P.O. Box 9517, 2300 RA Leiden, The Nether- lands. Email: [email protected] (corresponding author) 2 Netherlands Centre for Biodiversity Naturalis (section Zoological Museum of Amsterdam), Mauritskade 57, 1092 AD Amsterdam, The Netherlands. Email: [email protected] Abstract Part of the genus Lioconcha Mörch, 1853 is reviewed. Species strongly resembling Lioconcha castrensis (Linnaeus, 1758) are discussed and two new species are described: Lioconcha arabaya n. sp. from the Northwest Indian Ocean and Lioconcha rumphii n. sp. from Thailand and Sumatra. These three species, together with Lioconcha macaulayi Lamprell & Healy, 2002, share many morphological similarities and we suspect them to be closely related. They are referred to as the Lioconcha cast- rensis species group. Furthermore, lectotypes of Venus castrensis Linnaeus, 1758, and Venus fulminea Röding, 1798, are desig- nated. The latter is considered a junior synonym of V. castrensis. Key words: Indo-Pacific, Mollusca, Persian Gulf, Red Sea, taxonomy Introduction between the anterior and posterior extremities, height is measured vertically from the umbo to the ventral margin and The delimitation within the tropical venerid genus Lioconcha total width (or inflation) is the greatest distance between the Mörch, 1853, is problematic, due to high levels of external surfaces of the paired valves. For an extensive list of intraspecific morphological variability and relatively few synonyms of figured specimens of Lioconcha castrensis we useful morphological characters (Lamprell and Healy 2002). -

National Monitoring Program for Biodiversity and Non-Indigenous Species in Egypt
UNITED NATIONS ENVIRONMENT PROGRAM MEDITERRANEAN ACTION PLAN REGIONAL ACTIVITY CENTRE FOR SPECIALLY PROTECTED AREAS National monitoring program for biodiversity and non-indigenous species in Egypt PROF. MOUSTAFA M. FOUDA April 2017 1 Study required and financed by: Regional Activity Centre for Specially Protected Areas Boulevard du Leader Yasser Arafat BP 337 1080 Tunis Cedex – Tunisie Responsible of the study: Mehdi Aissi, EcApMEDII Programme officer In charge of the study: Prof. Moustafa M. Fouda Mr. Mohamed Said Abdelwarith Mr. Mahmoud Fawzy Kamel Ministry of Environment, Egyptian Environmental Affairs Agency (EEAA) With the participation of: Name, qualification and original institution of all the participants in the study (field mission or participation of national institutions) 2 TABLE OF CONTENTS page Acknowledgements 4 Preamble 5 Chapter 1: Introduction 9 Chapter 2: Institutional and regulatory aspects 40 Chapter 3: Scientific Aspects 49 Chapter 4: Development of monitoring program 59 Chapter 5: Existing Monitoring Program in Egypt 91 1. Monitoring program for habitat mapping 103 2. Marine MAMMALS monitoring program 109 3. Marine Turtles Monitoring Program 115 4. Monitoring Program for Seabirds 118 5. Non-Indigenous Species Monitoring Program 123 Chapter 6: Implementation / Operational Plan 131 Selected References 133 Annexes 143 3 AKNOWLEGEMENTS We would like to thank RAC/ SPA and EU for providing financial and technical assistances to prepare this monitoring programme. The preparation of this programme was the result of several contacts and interviews with many stakeholders from Government, research institutions, NGOs and fishermen. The author would like to express thanks to all for their support. In addition; we would like to acknowledge all participants who attended the workshop and represented the following institutions: 1. -

Community-Defined Research Priorities
Journal Pre-proof Fundamental questions and applications of sclerochronology: Community-defined research priorities Tamara Trofimova, Stella J. Alexandroff, Madelyn Mette, Elizabeth Tray, Paul G. Butler, Steven Campana, Elizabeth Harper, Andrew L.A. Johnson, John R. Morrongiello, Melita Peharda, Bernd R. Schöne, Carin Andersson, C. Fred T. Andrus, Bryan A. Black, Meghan Burchell, Michael L. Carroll, Kristine L. DeLong, Bronwyn M. Gillanders, Peter Grønkjær, Daniel Killam, Amy L. Prendergast, David J. Reynolds, James D. Scourse, Kotaro Shirai, Julien Thébault, Clive Trueman, Niels de Winter PII: S0272-7714(20)30708-3 DOI: https://doi.org/10.1016/j.ecss.2020.106977 Reference: YECSS 106977 To appear in: Estuarine, Coastal and Shelf Science Received Date: 1 February 2020 Revised Date: 15 July 2020 Accepted Date: 4 August 2020 Please cite this article as: Trofimova, T., Alexandroff, S.J., Mette, M., Tray, E., Butler, P.G., Campana, S., Harper, E., Johnson, A.L.A., Morrongiello, J.R., Peharda, M., Schöne, B.R., Andersson, C., Andrus, C.F.T., Black, B.A., Burchell, M., Carroll, M.L., DeLong, K.L., Gillanders, B.M., Grønkjær, P., Killam, D., Prendergast, A.L., Reynolds, D.J., Scourse, J.D., Shirai, K., Thébault, J., Trueman, C., de Winter, N., Fundamental questions and applications of sclerochronology: Community-defined research priorities, Estuarine, Coastal and Shelf Science (2020), doi: https://doi.org/10.1016/j.ecss.2020.106977. This is a PDF file of an article that has undergone enhancements after acceptance, such as the addition of a cover page and metadata, and formatting for readability, but it is not yet the definitive version of record. -

National Monitoring Program for Biodiversity and Non-Indigenous Species in Egypt
National monitoring program for biodiversity and non-indigenous species in Egypt January 2016 1 TABLE OF CONTENTS page Acknowledgements 3 Preamble 4 Chapter 1: Introduction 8 Overview of Egypt Biodiversity 37 Chapter 2: Institutional and regulatory aspects 39 National Legislations 39 Regional and International conventions and agreements 46 Chapter 3: Scientific Aspects 48 Summary of Egyptian Marine Biodiversity Knowledge 48 The Current Situation in Egypt 56 Present state of Biodiversity knowledge 57 Chapter 4: Development of monitoring program 58 Introduction 58 Conclusions 103 Suggested Monitoring Program Suggested monitoring program for habitat mapping 104 Suggested marine MAMMALS monitoring program 109 Suggested Marine Turtles Monitoring Program 115 Suggested Monitoring Program for Seabirds 117 Suggested Non-Indigenous Species Monitoring Program 121 Chapter 5: Implementation / Operational Plan 128 Selected References 130 Annexes 141 2 AKNOWLEGEMENTS 3 Preamble The Ecosystem Approach (EcAp) is a strategy for the integrated management of land, water and living resources that promotes conservation and sustainable use in an equitable way, as stated by the Convention of Biological Diversity. This process aims to achieve the Good Environmental Status (GES) through the elaborated 11 Ecological Objectives and their respective common indicators. Since 2008, Contracting Parties to the Barcelona Convention have adopted the EcAp and agreed on a roadmap for its implementation. First phases of the EcAp process led to the accomplishment of 5 steps of the scheduled 7-steps process such as: 1) Definition of an Ecological Vision for the Mediterranean; 2) Setting common Mediterranean strategic goals; 3) Identification of an important ecosystem properties and assessment of ecological status and pressures; 4) Development of a set of ecological objectives corresponding to the Vision and strategic goals; and 5) Derivation of operational objectives with indicators and target levels. -

Growth and Morphometric Characteristic of the Bivalve Callista Chione Population in Timsah Lake, Suez Canal, Egypt
CATRINA (2017), 16 (1):33-42 © 2017 BY THE EGYPTIAN SOCIETY FOR ENVIRONMENTAL SCIENCES Growth and Morphometric Characteristic of the Bivalve Callista chione Population in Timsah Lake, Suez Canal, Egypt Abdel-Fattah A. Ghobashy1, Mohamed H. Yassien2, Esraa E. AbouElmaaty2* 1Zoology Department, Faculty of Science, Suez Canal University, Ismailia, Egypt 2Invertebrates Aquaculture Laboratory, Aquaculture Division, National Institute of Oceanography and Fisheries, Gulfs of Suez and Aqaba branch, Attaqa, Suez, Egypt ABSTRACT This is the first attempt to study some biological aspects for the bivalve Callista chione in Egypt. The study of dimensional relationships assumes great importance in fishery biology researches. Studying the biological characteristics of C. chione is also essential for improving the state of current production and fishery management, as well as a base for introduction of its potential aquaculture. The growth of C. chione in Timsah Lake was studied in the period from June 2013 to August 2014 by the comparison of the rate of increase of one body parameter relative to that of the other parameter (allometry). The population characteristics of C. chione in Timsah Lake were studied depending on size frequency distribution to determine different age cohorts, growth parameters and mortality and exploitation rates. The results indicated that all morphometric relationships of C. chione showed a negative allometry. The length frequency analysis using FiSAT showed that C. chione population in the lake includes three age groups. The von Bertalanffy Growth Parameters; L∞, k and to, were 6.25 cm, 0.530 and -0.68 y. The growth performance index was estimated as 1.316. The natural mortality, fishing mortality and total mortality were found to be 0.5, 1.91 and 2.41 year-1. -

Monitoring of Infections by Protozoa of the Genera Nematopsis, Perkinsus
DISEASES OF AQUATIC ORGANISMS Vol. 42: 157–161, 2000 Published August 31 Dis Aquat Org NOTE Monitoring of infections by Protozoa of the genera Nematopsis, Perkinsus and Porospora in the smooth venus clam Callista chione from the North-Western Adriatic Sea (Italy) G. Canestri-Trotti1,*, E. M. Baccarani1, F. Paesanti2, E. Turolla2 1Dipartimento di Biologia Animale e dell’Uomo, Università degli Studi di Torino, Via Accademia Albertina, 17, 10123 Torino, Italy 2Goro Acquicoltura s.r.l., P. le Leo Scarpa, 45, 44020 Goro (Ferrara), Italy ABSTRACT: Marketable smooth venus clams Callista chione size (50 to 65 mm) were collected for a total of 375 from natural banks of Chioggia (Venice) and Goro (Ferrara), specimens (aged 4 to 6 yr after Marano et al. 1998): North-Western Adriatic Sea (Italy), were examined for proto- 357 specimens from Chioggia (Venice) and 18 speci- zoan parasites from November 1996 to November 1998. Out 2 of the 375 bivalves examined, 149 (39.7%) were infected by mens from Goro (Ferrara) (Table 1). Sections (1 cm ) of Nematopsis sp. and 325 (86.7%) by Porospora sp. Oocysts of gill, mantle and foot tissues were squashed between Nematopsis sp. were present with a prevalence that varied glass slides and examined for the presence of Nema- from 100% in November 1996 to 5% in June 1998; cystic and topsis (Apicomplexa: Porosporidae) oocysts and Poro- naked sporozoites of Porospora sp. were very common, with a prevalence of 100%. Out of the 229 bivalves examined spora (Apicomplexa: Porosporidae) sporozoites (Bower between January and November 1998, 63 (27.5%) were also et al. -

A Mortality Event of the Venerid Bivalve Callista Chione (Linnaeus, 1758) in a Hatchery a System - Case Study
""l Bull. Eur. Ass. Fish Patho1., 27(6) 2007, 214 A mortality event of the venerid bivalve Callista chione (Linnaeus, 1758) in a hatchery A system - case study M. Delgado*, N. Carrasco, L. Elandaloussi, D. Furones and A. Roque Institut de Recerca i Tecnologia Agroalimentaries (IRTA) Ctra. de Poble Nou, Km 5,5, E-43540 Sant CarIes de la Rapita, Tarragona, Spain. Abstract Abnormal mortality of the smooth venus clam (Callista chione) was encountered when conditioning these clams in a hatchery system. A histopathological analysis was performed to establish the causes of this mortality episode. Our results showed an increase in rickettsia-like bacteria infection intensity between the individuals collected at the start of the conditioning in the hatchery and those collected during the mortality episode. Husbandry stress most likely increased disease susceptibility and progression in these clams. Rickettsia-like colonies were observed in large numbers in the gills of all individuals examined. Nematopsis sp. spores and rod-shaped basophilic bacteria could also been seen in some of the individuals examined. Microbiological analysis of clam tissue did not reveal the presence of any potentially pathogenic bacteria and all the clams were shown to be free of Perkinsus sp. parasites. The conditioning protocol was adapted from those. used for other venerid clams due to the lack of data on this species. These findings highlight the need to perform further studies to evaluate the optimal parameters for C. chione broodstock conditioning. Introduction health status of C. chione appears crucial for The venerid bivalve Callista (=Cytherea) chione the development of hatchery-reared smooth (Linnaeus, 1758), or smooth clam, is clam and seed production. -
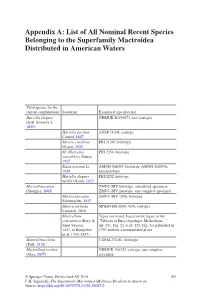
List of All Nominal Recent Species Belonging to the Superfamily Mactroidea Distributed in American Waters
Appendix A: List of All Nominal Recent Species Belonging to the Superfamily Mactroidea Distributed in American Waters Valid species (in the current combination) Synonym Examined type material Harvella elegans NHMUK 20190673, two syntypes (G.B. Sowerby I, 1825) Harvella pacifica ANSP 51308, syntype Conrad, 1867 Mactra estrellana PRI 21265, holotype Olsson, 1922 M. (Harvella) PRI 2354, holotype sanctiblasii Maury, 1925 Raeta maxima Li, AMNH 268093, lectotype; AMNH 268093a, 1930 paralectotype Harvella elegans PRI 2252, holotype tucilla Olsson, 1932 Mactrellona alata ZMUC-BIV, holotype, articulated specimen; (Spengler, 1802) ZMUC-BIV, paratype, one complete specimen Mactra laevigata ZMUC-BIV 1036, holotype Schumacher, 1817 Mactra carinata MNHN-IM-2000-7038, syntypes Lamarck, 1818 Mactrellona Types not found, based on the figure of the concentrica (Bory de “Tableau of Encyclopedique Methodique…” Saint Vincent, (pl. 251, Fig. 2a, b, pl. 252, Fig. 2c) published in 1827, in Bruguière 1797 without a nomenclatorial act et al. 1791–1827) Mactrellona clisia USNM 271481, holotype (Dall, 1915) Mactrellona exoleta NHMUK 196327, syntype, one complete (Gray, 1837) specimen © Springer Nature Switzerland AG 2019 103 J. H. Signorelli, The Superfamily Mactroidea (Mollusca:Bivalvia) in American Waters, https://doi.org/10.1007/978-3-030-29097-9 104 Appendix A: List of All Nominal Recent Species Belonging to the Superfamily… Valid species (in the current combination) Synonym Examined type material Lutraria ventricosa MCZ 169451, holotype; MCZ 169452, paratype; -

STATE of BIODIVERSITY in the MEDITERRANEAN (2-3 P
UNEP(DEC)/MED WG.231/18 17 April 2003 ENGLISH MEDITERRANEAN ACTION PLAN Meeting of the MED POL National Coordinators Sangemini, Italy, 27 - 30 May 2003 STRATEGIC ACTION PROGRAMME GUIDELINES DEVELOPMENT OF ECOLOGICAL STATUS AND STRESS REDUCTION INDICATORS FOR THE MEDITERRANEAN REGION In cooperation with UNEP Athens, 2003 TABLE OF CONTENTS Pages 1. INTRODUCTION ......................................................................................................... 1 2. AIMS OF THE REPORT .............................................................................................. 2 3. STATE OF BIODIVERSITY IN THE MEDITERRANEAN............................................. 2 Species Diversity................................................................................................................. 2 Ecosystems/Communities .................................................................................................. 3 Pelagic ............................................................................................................................... 3 Benthic ............................................................................................................................... 4 4. ECOSYSTEM CHANGES DUE TO ANTHROPOGENIC IMPACT............................... 6 Microbial contamination...................................................................................................... 6 Industrial pollution .............................................................................................................. 6 Oil -

DNA Barcoding Reveal Patterns of Species Diversity Among
www.nature.com/scientificreports OPEN DNA barcoding reveal patterns of species diversity among northwestern Pacific molluscs Received: 04 April 2016 Shao’e Sun, Qi Li, Lingfeng Kong, Hong Yu, Xiaodong Zheng, Ruihai Yu, Lina Dai, Yan Sun, Accepted: 25 August 2016 Jun Chen, Jun Liu, Lehai Ni, Yanwei Feng, Zhenzhen Yu, Shanmei Zou & Jiping Lin Published: 19 September 2016 This study represents the first comprehensive molecular assessment of northwestern Pacific molluscs. In total, 2801 DNA barcodes belonging to 569 species from China, Japan and Korea were analyzed. An overlap between intra- and interspecific genetic distances was present in 71 species. We tested the efficacy of this library by simulating a sequence-based specimen identification scenario using Best Match (BM), Best Close Match (BCM) and All Species Barcode (ASB) criteria with three threshold values. BM approach returned 89.15% true identifications (95.27% when excluding singletons). The highest success rate of congruent identifications was obtained with BCM at 0.053 threshold. The analysis of our barcode library together with public data resulted in 582 Barcode Index Numbers (BINs), 72.2% of which was found to be concordantly with morphology-based identifications. The discrepancies were divided in two groups: sequences from different species clustered in a single BIN and conspecific sequences divided in one more BINs. In Neighbour-Joining phenogram, 2,320 (83.0%) queries fromed 355 (62.4%) species-specific barcode clusters allowing their successful identification. 33 species showed paraphyletic and haplotype sharing. 62 cases are represented by deeply diverged lineages. This study suggest an increased species diversity in this region, highlighting taxonomic revision and conservation strategy for the cryptic complexes. -

(Marlin) Review of Biodiversity for Marine Spatial Planning Within
The Marine Life Information Network® for Britain and Ireland (MarLIN) Review of Biodiversity for Marine Spatial Planning within the Firth of Clyde Report to: The SSMEI Clyde Pilot from the Marine Life Information Network (MarLIN). Contract no. R70073PUR Olivia Langmead Emma Jackson Dan Lear Jayne Evans Becky Seeley Rob Ellis Nova Mieszkowska Harvey Tyler-Walters FINAL REPORT October 2008 Reference: Langmead, O., Jackson, E., Lear, D., Evans, J., Seeley, B. Ellis, R., Mieszkowska, N. and Tyler-Walters, H. (2008). The Review of Biodiversity for Marine Spatial Planning within the Firth of Clyde. Report to the SSMEI Clyde Pilot from the Marine Life Information Network (MarLIN). Plymouth: Marine Biological Association of the United Kingdom. [Contract number R70073PUR] 1 Firth of Clyde Biodiversity Review 2 Firth of Clyde Biodiversity Review Contents Executive summary................................................................................11 1. Introduction...................................................................................15 1.1 Marine Spatial Planning................................................................15 1.1.1 Ecosystem Approach..............................................................15 1.1.2 Recording the Current Situation ................................................16 1.1.3 National and International obligations and policy drivers..................16 1.2 Scottish Sustainable Marine Environment Initiative...............................17 1.2.1 SSMEI Clyde Pilot ..................................................................17 -

Mollusca: Bivalvia) on the Catalan Coast (NE Spain
ISSN: 0001-5113 ACTA ADRIAT., ORIGINAL SCIENTIFIC PAPER AADRAY 57(1): 93 - 106, 2016 Broodstock conditioning and gonadal development of the smooth clam Callista chione (Linnaeus, 1758) (Mollusca: Bivalvia) on the Catalan coast (NE Spain) Marina DELGADO1*, Josu PÉREZ-LARRUSCAIN2 and Joan IGNASI GAIRÍN2 1Instituto Español de Oceanografía. Centro Oceanográfico de Cádiz. Muelle de Levante s/n, P.O. Box 2609. E-11006 Cádiz (Spain) 2Institut de Recerca i Tecnologia Agroalimentaries (IRTA). Centre de Aqüicultura. Carretera del Poble Nou, Km 5.5. E-43540 Sant Carles de la Rápita, Tarragona (Spain) *Corresponding author, e-mail: [email protected] In order to select the most suitable broodstock conditions for Callista chione, a conditioning experiment was performed and the gametogenic cycle of a natural population on the coast of Arenys de Mar (Catalonia, NE Spain) was studied. The influence of food availability and sand bed presence on energy balance and gonadal development was analyzed, also the proliferation of intracellular bacteria in gills was monitored. The gametogenic cycle started between August and September. In November, most of the gonads were ripe and spawning began in December. The higher abundance of spawning clams occurred between January and April (with sea surface temperature 13-15ºC). Three experimental conditions were tested in September at a constant temperature of 14ºC:T1 (0.10% of organic weight of microalgae as a proportion of the live weight of the clams per day and a sand bed); T2 (0.05% and a sand bed); T3 (0.10% without a sand bed). Gonadal maturity was a priority for this species and was reached at the end of all trials.