Researcher 2015;7(10)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Coca Cola out of South Africa, but Is It the Real Thing?
Number Three The newsletter of Washington's STATE-WIDE ANTI-APARTHEID NETWORK COCA COLA OUT OF SOUTH AFRICA, BUT IS IT THE REAL THING? Inside this issue of SWAAN Call: The latest on the Coca-Cola campaign (page two) Congress passes historic sanctions bill (page 11) National day of protest on 10 October (page 3) PLUS: Regional updates, October/November Freedom Calendar, and more . .. Products of DOES APARTHEID The Coca-Cola Company Coca-Cola (classic, diet, cherry, etc.) GO BETTER WITH TAB Sprite Mello Yello Fresca Mr. PIBB Hi-C soft drinks Fanta Five-Alive COKE? Minute Maid Ju~ces Ramblin' root beer Bright and Early beverages Maryland Club coffee The Coca-Cola Company controls 90 percent Butter- Nut coffee of t he soft drink market in South Africa, Belmont Springs distilled water and is the third largest employer there, with 5,000 employees. Columbia Pictures Tri-Star Pictures (partial ownership) The company announced on 17 September that Embassy Television it c.Jould "disinvest" by selling its hold RCA/Columbia Pictures Home Video ings to black South African businessmen, Walter Reade theatres so the public was confused that the Georgia Coalition for Divestment did not *********************************** cancel plans to launch a nationwide Coke What the Coca-Cola Company has to say: Divestment Campaign on 10 October. WHY? The goal is to pressure Coca-Cola into "We have committed $10 million to the leading corporate withdrawal from South Equal Opportunity Funds, independent Sou~h Africa. This has not happened. African foundations which we are confident will play a major role in the shaping of "For one thing," according to the Wall post-apartheid South Africa. -

Cocktails 11.5 BEER & CIDER
Wines 11.5 Cocktails SPARKLING NIGHTLY COCKTAIL CAVIT LUNETTA PROSECCO SPLIT VENETO, IT 11 BARTENDER’S CHOICE SEGURA VIUDAS BRUT ROSE SPLIT CAVA, CA 11 DUKE OF RAOUL WOODY CREEK VODKA, DOLIN BLANC, HOUSE SMOKED BACON PRINCE DE RICHEMONT DEMI-SEC BOTTLE, FR 40 MOUNT RANIER HIGHWAY TATTOOSH BOURBON, LONG ROAD HARD CIDER, ORANGE WHITES THE DEACON ST. URBANS-HOF OLD VINES RIESLING, GERMANY 12/44 KETEL ONE ORANGE VODKA, HOUSE LEMONADE, ICED TEA AU CONTRAIRE CHARDONNAY RUSSIAN RIVER VALLEY, CA 12/44 Five alive IL CONTI PINOT GRIGIO FOSSALTA DI PIAVE, IT 10/36 AVIATOR GIN, GRAPEFRUIT, LEMON, LIME, ORANGE, PROSECCO SAINTE ROSELINE PERLE ROSE, FRANCE 10/36 M-TOWN MULE OLD DOMINIC HONEYBELL VODKA, LIME, GOSLING’S GINGER BEER PETER YEALANDS SAUVIGNON BLANC MARLBOROUGH, NZ 12/44 THREE-EIGHT-ONE-OH!-FLOOR A TO Z WINEWORKS CHARDONNAY, OREGON 10/36 UV BLUE RASPBERRY VODKA, BLANCO TEQUILA, WOODY CREEK GIN, BACARDI RUM DUCKHORN CHARDONNAY HOPLAND, CA 95 OLDE TYMES COOPER’S CRAFT BOURBON, ELDERFLOWER, BITTERS, ORANGE, CHERRY REDS SEGA GENESIS TITO’S VODKA, FRESH LIMEADE, CHERRIES VINUM PETITE SYRAH OAKVILLE, CA 10/36 BEER & CIDER ANNE AMIE PINOT NOIR WILLAMETTE VALLEY, OR 12/44 ROTATING SELECTION ON DRAFT 6.5 HESS CABERNET SAUVIGNON POPE VALLEY, CA 12/44 THE PATH MERLOT SONOMA, CA 10/36 cans & bottles CHATEAU DE BELLEVUE LUSSAC SAINT ÉMILION BORDEAUX, FR 12/44 Budweiser 4.5 GUNSIGHT ROCK CABERNET PASO ROBLES, CA 11/36 Miller Lite 4.5 PULENTA LA FLOR MALBEC, ARGENTINA 12/44 Southern Prohibition Blonde 5.5 ETUDE CARNEROS PINOT NOIR NAPA VALLEY, CA 14/52 Bud Light 4.5 HERITANCE CABARNET SAUVIGNON SAINT HELENA, CA 95 Stella Artois 5.5 Blue Moon 4.5 Goldcrest 5.5 BOTTLE SPECIAL 25 Shiner Bock Blonde 4.5 Michelob Ultra 4.5 Lagunitas IPA 5.5 Pabst Blue Ribbon 16oz 4.5 Ghost River Golden Ale 5.5 Coors Light 4.5 Buckler Non-Alcoholic 4.5 . -

To Start Tacos to Share
TO START TACOS served on 4" flour tortillas Char Roasted Pacific Shrimp Charred Rapini black chili oil, pineapple, lime juice, cilantro 12 romanesca salsa, almonds 3.75 Tomato Gazpacho Sweet Potato and Leek grilled scallop, cucumber, jicama, red onion 8 chimichurri, queso fresco 3.75 Grilled Romaine Grilled Squid roasted tomato, avocado, radish, hibiscus egg, pickled crispy coconut, shredded cabbage, tamarind mayo, onion 11 + grilled pork belly 4 jalapeños 3.75 Esquites Grilled Skirt Steak roasted corn, chipotle mayo, lime, cilantro, cotija, house grilled onions, roasted tomatillo salsa 4.25 chili mix 6 Grilled Chicken Thighs Queso Fundido mole, pineapple, pickled white onions, cilantro 3.75 melted cheese, nopales, pickled onions, tortillas 14 + green chorizo 5 Crispy Cod jalapeño avo crema, pickled onion, cilantro, radish 3.75 Chilaquilles crispy tortillas, roasted tomatillo salsa, pickled onions, mushrooms, soft egg 6 + green chorizo 5 + black beans 3 TO SHARE served with 4"flour tortillas Tuna Ceviche Whole Mackerel tomato, mango, salsa fresca water, cucumber, onion 12 tomatillo, cucumber, cilantro, pickled onions, radishes, crispy shallots 28 Chips & Dips choose three guacamole, salsa fresca, spiced eggplant, roasted Half Chicken vegetable salsa, black bean 15 yucatan spiced salsa, tamarind mayo slaw, avocado, cilantro 27 Bean and Cheese Empanadas guacamole, verde, lime crema 8 7oz Top Sirloin chimichurri, crispy garlic, pickled pumpkin, fried cheese Nachos curds 30 roasted jalapeños, feta, monterey jack, salsa fresca, chipotle crema, -

The Effect of Ph and Chemical Preservatives on the Growth of Bacterial Isolates from Commercial Samples of Fruit Juices Sold in South Eastern Nigeria
International Journal of Research Studies in Biosciences (IJRSB) Volume 3, Issue 10, October 2015, PP 141-145 ISSN 2349-0357 (Print) & ISSN 2349-0365 (Online) www.arcjournals.org The Effect of pH and Chemical Preservatives on the Growth of Bacterial Isolates from Commercial Samples of Fruit Juices Sold in South Eastern Nigeria Onyeneto, T.C. Nwachukwu, I.N, Nwogwugwu, N.U Department of Microbiology, Anambra Department of Microbiology, Federal State University of Science and University of Technology, Owerri, Nigeria. Technology, Uli, Nigeria. [email protected] Abstract: Five bacterial pathogens were isolated from commercial samples of fruit juices sold in South Eastern Nigeria. The isolates were characterized and identified as Bacillus, Staphylococcus, Psuedomonas, Lactobacillus and Gluconobacter species. The effect of pH, benzoic acid and Sodium chloride concentration on the growth rate of isolates was investigated. The result was that as the pH of the growth medium increased from 3 to 7, the rate of growth of the isolates increased. As the concentration of Sodium chloride increased from 2 to 4%, the rate of growth of the isolated decreased. As the concentration of benzoic acid increased from 250 to 1000mg/L, to growth rate of all the isolates decreased. Also as the concentration of Sodium chloride increased from 2 to 5% the growth rate of all the isolates decreased. The higher the concentration of the preservatives the lower the rate of growth of the isolates. Key words: Preservatives, Bacillus, Staphylococcus, Psuedomonas, Lactobacillus and Gluconobacter species 1. INTRODUCTION Fruit juice is extractable liquid naturally contained in fruit or vegetable tissue. Fruit juices are essentially products from fresh fruits such as orange, mango, pineapple, grapes etc. -

A Beverage Company Coca-Colacompany.Com
A beverage company Coca-colacompany.com Backgrounder The Coca-Cola Company The Coca-Cola Company began in 1886 in Atlanta, GA when Dr. John S. Pemberton, a pharmacist, created a soft drink with flavored syrup and carbonated water. Frank M. Robinson, his partner, thought of the name “Coca-Cola.” He thought that the two Cs would sound great together. Robinson also created the unique and famous script that is still used today. Coca-Cola was first sold in an Atlanta pharmacy. The popularity grew and was expanded outside of Atlanta. By 1894, Coca-Cola was distributed in bottles. In 1944, Coca-Cola became a registered trademark. The creative and distinctive Coca-Cola bottle was trademarked in 1977. From 1886 to now, Coca-Cola has the world’s most beloved beverages. Mission and Values The mission of Coca-Cola is to refresh the world, to inspire moments of optimism and happiness and to create value and make a difference. The company provides a great working environment, a quality of beverage brands, a network of customers and suppliers, and social responsibility by helping build and support sustainable communities. Coca-Cola is focused on reducing its environmental footprint and supporting active, healthy living. Coca-Cola values leadership, collaboration, integrity, accountability, passion, diversity and quality. Products Today, the Coca-Cola Company is the world’s largest beverage company. The company has more than 500 sparkling and still brands. Twenty of those are billion- dollar brands including Fanta, Sprite, Diet Coke, Coca-Cola zero, vitaminwater, POWERADE, Simply, Minute Maid, Georgia, Dasani, FUZE TEA and Del Valle. -

Nutrition Tips
Nutrition Resources The following pages are a few resources provided to Team Ontario Beach by our dieticians at the Canadian Sports Institute of Ontario that we would like to share with the community to assist in helping club and community athletes best prepare themselves for optimal training and competition. • Balanced Eating for Optimal Performance • Athlete’s Training Day Plates • A few Recipes for Before, During, After, Exercise Our current dietician is Nicole Springle, RD, the Lead of Sport Nutrition at the Canadian Sport Institute of Ontario. (www.csiontario.ca) Balanced Eating for Optimal Energy & Performance: Combine Protein and Carbohydrates Choose meals and snacks that combine protein and carbohydrate for lasting energy. The balance can keep you full longer, help reduce cravings, increase energy, and help keep you from overeating at your next meal. For best results, you should generally avoid going longer than four hours without eating, and look for a minimum of 15-20g of protein at each meal and 5-10g at each snack. Size may vary depending on activity level. CARBOHYDRATE PROTEIN Grains and Starches (highest carb): Meats (highest protein): ñ Breads, bagels, buns (whole wheat or whole grain) ñ Fish (avoid deep-fried) ñ Tortilla/fajita shells (same) ñ Shellfish (avoid deep-fried) ñ Pitas (same) ñ Turkey (choose skinless, white, roasted more often) ñ Crackers (choose whole grain, trans fat-free) ñ Chicken (same) ñ Pasta (choose whole grain over white) ñ Pork/Ham (choose lean cuts, avoid sausages and ñ Rice (choose brown or wild over white) processed meats) ñ Potatoes, sweet potatoes, yams, etc. -

General Nutrition Guidelines for Hemodialysis July 2011
General Nutrition Guidelines for Hemodialysis July 2011 Salt/Sodium Salt is only needed in small amounts. Limit salt in your diet. Salt is found naturally in food and water Salt is added to foods in processing Salt can increase thirst and increase dialysis fluid gains. Salt can also increase your blood pressure. Avoid using salt in cooking and at the table. Limit your intake of salty foods. Lower Salt Items Higher Salt items Good choices Limit/Avoid Flavour/Seasonings Cheese Flavour/Seasonings Cheese Herbs Cream cheese Seasoning salt Processed cheese Spices Brick cheese Celery salt Cheese Whiz® Black pepper Brie Garlic salt Fresh garlic Swiss Lemon pepper Meats/Alternatives Fresh onion Mozzarella Table salt Canned meats Lemon/lime Ricotta Sea salt Bacon Vinegar Havarti Ketchup Salami Vanilla extract Monterey Oyster/fish sauce Bologna Mrs. Dash® Colby Mustard Sausages McCormick’s No Salt Gruyere Soy sauce Wieners added ® BBQ sauce Sardines Meats/Alternatives Relish Pepperoni, Fresh poultry Worcestershire sauce Spam®/Klik® Fresh beef Ham Fresh pork Other Fish Instant Noodles Snacks Eggs Rice/pasta noodle mixes Salted snacks Homemade Rinsed canned tuna Rinsed Kraft Dinner® Pretzels Soups canned salmon Hamburger Helper® Instant Potato chips Pancakes Wild meat cereals Microwave popcorn Waffles Alphagetti® Tortilla chips Muffins Other Packaged gravies Chips & dip Air popped popcorn Canned gravies/sauces Breads/Cereal Unsalted crackers Shake and Bake® Soup Bread Unsalted pretzels Fast foods Canned soups Buns Homemade gravies TV dinners Bouillon Rice Homemade sauces Pickles Consommé Pasta Tomato/V8 ® juice Dry soup mixes Cold & hot cereals *Do not use salt substitutes, such as No Salt® & Half Salt® as they are high in Potassium. -

11. the Life Cycle of Food
Secondary School, Cycle 1 11. The Life Cycle of Food Duration: 75 min. Far from innocuous, eating is one of our daily activities that has the biggest impact on the environment. Through examining the life cycle of various foods from the field to our plates, the students discover the 3N’s-F principle (Non-packaged, Not far, Natural and Fair). They are then asked to put these principles into practice as a way to make healthier, more ecological and fairer food choices. In so doing, they become more enlightened consumers! Learning Objectives Preparation Have students: Become familiar with the 3N’s-F principle Develop their critical sense by examining by reading the book L’envers de l’assiette and comparing the impact of a range of et quelques idées pour la remettre à food items on our health, the environ- l’endroit (optional – see References). ment and society. Become familiar with the content of the Become aware of how the conventional appendices and print copies of them food system works and its complexities. according to the number of students. Learn about the main stages in the life Bring in the products specified in cycle of food. Appendix 1.1 (or cut out the product Become aware of the purchasing power descriptions)*. of the enlightened consumer (activist). Prepare the material for the students (cut out the life cycle stages in Appendix Areas of Learning 2 for each of the teams). English, science, biology and geography. Materials 1 copy per team of the “3N’s-F” chart (Appendix 1.2) and the “Life Cycle of Food” summary (Appendix 2). -

Coca-Cola Ltd. 335 King Street East, Toronto, Ontario, M5A 1L1
Canadian Children’s Food and Beverage Advertising Initiative: The Coca-Cola Company’s Commitment FINAL Section A -- Identifying Information The corporate name and address of the participant: Coca-Cola Ltd. 335 King Street East, Toronto, Ontario, M5A 1L1 Point of contact for implementation of Initiative: Shannon C. Denny Director, Public Affairs Communications 416-424-6373 [email protected] This commitment covers all brands owned by Coca-Cola Ltd. Canada, including: Coca-Cola Coca-Cola Zero Cherry Coke Diet Coke Vanilla Coke Barq’s Diet Barq’s Sprite Sprite Zero Fanta Fresca Five Alive Minute Maid Simply Nestea glaceau vitaminwater glaceau smartwater Dasani Odwalla Fruitopia Powerade Core Power FUZE ZICO May 2016 Section B -- Core Principles – Coca-Cola Canada’s commitment to Responsible Marketing Coca-Cola Ltd. recognizes the positive and important role it can play in helping to shape choice, and developing and promoting a variety of beverage choices for young people that provide refreshment, enjoyment, nutrition and hydration. To help us do that, we define and update our operating guiding principles from time to time. We listen to our customers as well as to our consumers, many of whom are parents, teachers, doctors and community leaders. In response to their needs, our current advertising policy reflects our commitment to support parents and other caregivers in their special roles as gatekeepers in all decisions affecting the lives of their children, including beverage choices. Accordingly, Coca-Cola Ltd. will not directly market to children under the age of 12. We firmly believe that all of our products are of the highest quality and suitable for all consumers. -

Appendix Unilever Brands
The Diffusion and Distribution of New Consumer Packaged Foods in Emerging Markets and what it Means for Globalized versus Regional Customized Products - http://globalfoodforums.com/new-food-products-emerging- markets/ - Composed May 2005 APPENDIX I: SELECTED FOOD BRANDS (and Sub-brands) Sample of Unilever Food Brands Source: http://www.unilever.com/brands/food/ Retrieved 2/7/05 Global Food Brand Families Becel, Flora Hellmann's, Amora, Calvé, Wish-Bone Lipton Bertolli Iglo, Birds Eye, Findus Slim-Fast Blue Band, Rama, Country Crock, Doriana Knorr Unilever Foodsolutions Heart Sample of Nestles Food Brands http://www.nestle.com/Our_Brands/Our+Brands.htm and http://www.nestle.co.uk/about/brands/ - Retrieved 2/7/05 Baby Foods: Alete, Beba, Nestle Dairy Products: Nido, Nespray, La Lechera and Carnation, Gloria, Coffee-Mate, Carnation Evaporated Milk, Tip Top, Simply Double, Fussells Breakfast Cereals: Nesquik Cereal, Clusters, Fruitful, Golden Nuggets, Shreddies, Golden Grahams, Cinnamon Grahams, Frosted Shreddies, Fitnesse and Fruit, Shredded Wheat, Cheerios, Force Flake, Cookie Crisp, Fitnesse Notes: Some brands in a joint venture – Cereal Worldwide Partnership, with General Mills Ice Cream: Maxibon, Extreme Chocolate & Confectionery: Crunch, Smarties, KitKat, Caramac, Yorkie, Golden Cup, Rolo, Aero, Walnut Whip, Drifter, Smarties, Milkybar, Toffee Crisp, Willy Wonka's Xploder, Crunch, Maverick, Lion Bar, Munchies Prepared Foods, Soups: Maggi, Buitoni, Stouffer's, Build Up Nutrition Beverages: Nesquik, Milo, Nescau, Nestea, Nescafé, Nestlé's -

Ananas Comosus) Drink Before and During Storage
Current Journal of Applied Science and Technology 30(1): 1-10, 2018; Article no.CJAST.44040 ISSN: 2457-1024 (Past name: British Journal of Applied Science & Technology, Past ISSN: 2231-0843, NLM ID: 101664541) Effects of Honey on Total Phenolic Content and Antioxidant Activities of Pineapple (Ananas comosus) Drink Before and During Storage T. K. Adebayo1*, I. A. Abdulraheem1, A. S. Daramola1 and T. C. Israel1 1Department of Food Technology, Federal Polytechnic, Offa, Kwara State, Nigeria. Authors’ contributions This work was carried out in collaboration among all authors. Author TKA designed the study. Author IAA carried out the experiment and the statistical analysis, while author ASD wrote the first draft of the manuscript. Authors TKA and TCI corrected the manuscript and confirmed the literatures. All the authors read and approved the final manuscript. Article Information DOI: 10.9734/CJAST/2018/44040 Editor(s): (1) Dr. Ming-Chih Shih, Professor, Department of Health and Nutrition Science, Chinese Culture University, Taiwan. (2) Dr. Hamid El Bilali, Centre for Development Research, University of Natural Resources and Life Sciences (BOKU), Vienna, Austria. Reviewers: (1) Rosendo Balois Morales, Universidad Autonoma de Nayarit, Mexico. (2) Camel Lagnika, School of Farm Product Conservation and Processing, National University of Agriculture, Benin. (3) Coulibaly Wahauwouele Hermann, University Nangui Abrogoua, Côte d’Ivoire. (4) Okudu Helen Ochanya, Michael Okpara University of Agriculture, Nigeria. Complete Peer review History: http://www.sciencedomain.org/review-history/26830 Received 21 August 2018 Original Research Article Accepted 02 October 2018 Published 25 October 2018 ABSTRACT This study determined the effects of honey on total phenolic content and antioxidant activities of pineapple (Ananas comosus) drink before and during storage. -
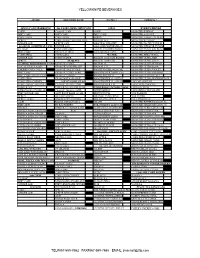
A1-AUG 03.Xlsx
YELLOWKNIFE BEVERAGES STORE DELIVERY DATE NOTES ? CREDITS ? 20X355 CANS SPARKLING 4x6 CASES 222ML MINI CANS 12X1L ENERGY DRINKS COKE CLASSIC CAN COKE MONSTER ENERGY DIET COKE DIET COKE DIET COKE MONSTER ASSAULT COKE ZERO COKE ZERO SPRITE MONSTER ABSOLUTE ZERO SPRITE COKE ZERO CAFFEINE FREE GINGER ALE MONSTER KHAOTIC GINGER ALE SPRITE CAN AHA PEACH HONEY MONSTER PACIFIC PUNCH 24x355ML OVERWRAP CANS GINGER ALE AHA LIME WATERMELON MONSTER PIPELINE PUNCH CLASSIC FANTA ORANGE AHA BLUEBERRY POMEG. MONSTER PAPILLION PUNCH DIET COKE FRESCA CITRUS AHA ORANGE GPFT. MONSTER MANGO LOCO COKE ZERO CLUB SODA WATER MONSTER ZERO ULTRA GINGER ALE TONIC WATER DASANI 12X355ML BOXED MONSTER ULTRA RED SPRITE 8X300 PET DASANI 24X591ML MONSTER ULTRA BLUE 12x355ML FRIDGEMATE CANS 8X300 PET COKE DASANI 1L MONSTER ULTRA SUNRISE COCA COLA CLASSIC 8X300 PET GINGER ALE DASANI 1.5L MONSTER ULTRA ROSA CHERRY COCA COLA 8X300 PET DIET COKE SMART WATER 24X591ML MONSTER ULTRA VIOLET DIET COKE 8X300 PET COKE ZERO SMARTWATER 700ML CAP MONSTER ULTRA PARADISE DIET COKE FEISTY CHERRY 8X300 PET SPRITE SMART WATER 1L MONSTER ULTRA FIESTA COKE ZERO 8X300 FANTA ORANGE SMARTWATER 1L ANTIOX MONSTER ULTRA WATERMELON COKE ZERO CHERRY 24X237-355ML GLASS SMARTWATER 1L ALKALINE JAVA MEAN BEAN COKE ZERO CAFFEINE FREE CLASSIC GLASS 237ML SMARTWATER 1.5L JAVA LOCHA MOCHA GINGER ALE GINGER ALE GLASS 237ML POWERADE 12x710ML/946ML JAVA OAT MILK LEMONADE GINGER ALE CLASSIC GLASS 330ML MIXED BERRY JAVA SWISS CHOCOLATE DIET GINGER ALE A&W ROOT BEER 341ML FRUIT PUNCH NOS PASSION SPRITE QUEBEC MAPLE