Tuberculosis-Associated Immune Reconstitution Inflammatory Syndrome: Case Definitions for Use in Resource-Limited Settings
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Decade of Change for Newborn Survival
A Decade of Change for Newborn Survival Changing the trajectory of our future Overview of the supplement and Uganda analysis Health Policy and Planning, Supplement 3, 2012 Dr Anthony K Mbonye Ministry of Health Outline of presentations 1. Overview of a decade of change supplement Dr Anthony Mbonye, Ministry of Health 2. Changes in newborn health outcomes and coverage indicators Dr Olive Sentumbwe, World Heath Organization 3. Programmatic and policy changes over the past decade Dr Hanifah Sengendo, Saving Newborn Lives, Save the Children 4. Changing the trajectory for our future Dr Gelasius Mukasa, IBFAN; Newborn Steering Committee Uganda Decade of Change and Future Implications Analysis Group Allisyn Moran (Saving Newborn Lives Save the Children) Anthony K Mbonye (Community Health Services Ministry of Health) Christine Zirabamuzaale (Consultant) Newborn health Francine Kimanuka (UNICEF Uganda) Gelasius K Mukasa (IBFAN) champions: Geofrey Bisoborwa (WHO Uganda) Hanifah Naamala Sengendo (Save the Children Uganda) 11 authors Imelda Namagembe (AOGU) on behalf of 30 Jamil Mugalu (Mulago Hospital) Janex M Kabarangira (USAID) person expert Jessica Nsungwa-Sabiiti (Ministry of Health) Joy E Lawn (Saving Newborn Lives Save the Children) working group Kate Kerber (Saving Newborn Lives Save the Children) Representing Lillian Luwaga (Ministry of Health) Margaret Nakakeeto (Child Health Advocacy International – Uganda) government, heath Mary Kinney (Saving Newborn Lives Save the Children) Miriam Mutabazi [Management Sciences for Health (MSH)] professional -

MLI Newsletter July.Pdf
Volume 2 Issue 3 July 2019 has also developed and implemented respiratory medicine training programs for primary health care providers and Dr. Bruce J Kirenga initiated super specialized skills training programs. In line with our mission, we opened a translational chest clinic Dear Readers, which offers clinical services found in very few centres in Africa such as a sleep disorders clinic and lab, pulmonary MLI organised a two-day event during which we held the 1st function testing, allergy testing and pulmonary rehabilitation International Lung Science Symposium and the institute’s among others. inauguration ceremony. As we celebrate this milestone, we look Moving forward, MLI will harness opportunities that exist back at some of MLIs achievements in addressing the problem while creating others, advocate for the inclusion of lung health of the lung diseases epidemic in Uganda such as preforming medicines in the essential medicines kits and expand its pioneer studies on air pollution (including indoor air pollution) collaboration base. and participating in national lung disease surveys such as the National asthma survey, National tuberculosis survey, chronic I wish you an enjoyable read obstructive pulmonary disease surveys, among others. MLI Science for healthy lungs as we build for the future Makerere University Lung Institute Inauguration Story on page 2 Makerere University held its inauguration ceremony on the 30th April 2109. The institute however has been in existence for four years. MLI was the brainchild of its current director, Dr. Bruce Kirenga, who worked alongside fellow lung health professionals in Mulago and abroad to start an institute that would bring more attention to chronic lung diseases. -

Thesis for Word XP
Thesis for doctoral degree (Ph.D.) 2009 Thesis for doctoral degree (Ph.D.) 2009 (Ph.D.) degree doctoral Thesis for Care of The Newborn in Uganda Studies of the use of simple affordable effective interventions Care ofCare in Uganda The Newborn Romano Nkumbwa Byaruhanga Nkumbwa Romano Romano Nkumbwa Byaruhanga From the Division of Global Health (IHCAR), Department of Public Health Sciences, Karolinska Institutet, Stockholm, Sweden CARE OF THE NEWBORN IN UGANDA Studies of the use of simple affordable effective interventions Romano Nkumbwa Byaruhanga Stockholm 2009 The cover picture shows a postpartum mother practicing Skin to Skin contact. The back picture depicts a traditional birth attendant homestead where deliveries are conducted. All previously published papers were reproduced with permission from the publishers. Published by Karolinska Institutet. Printed by Universitetsservice US-AB © Romano Nkumbwa Byaruhanga, 2009 ISBN 978-91-7409-705-4 “We must accept finite disappointment, but we must never lose infinite hope.” Martin Luther King Jr. ABSTRACT Background: There are evidence based cost effective interventions available, which could decrease neonatal mortality, if scaled up and delivered under ideal conditions. Aim: To determine the causes of perinatal deaths, risk factors for neonatal hypothermia and explore the acceptability and feasibility of recommended perinatal practices in hospital and community settings in Uganda. Settings: St. Raphael of St. Francis Hospital, Nsambya in Kampala and rural villages in Ntungamo, Kayunga and Soroti district. Methods: The study period was from 1997-2008. A data form with a checklist and structured written questionnaires were used to collect data for studies I, II, III. 235 hospital records of women who had experienced a perinatal death in study I were reviewed. -

Ashar Cheptoris the Reason Cheptoris Says She Single Mum, Who Raised Two Doctors Dental Surgeon Decided to Stay in the Country
Issue 66 June 2019 Arise A Women’s Development Magazine Published by ACFODE WOMEN IN UNIFORM: Traditional Versus None Traditional Careers for Women INCLUDES: TRADITIONAL VERSUS NON - TRADITIONAL CAREERS: WHAT INFLUENCES A WOMAN CAREER CHOICE? IS RAISING A FAMILY MISSION IMPOSSIBLE FOR WOMEN IN UNIFORM? A CASE OF THE UGANDA POLICE FORCE THE MEDICAL FIELD: HOW FEMALE HEALTH WORKERS HAVE CHANGED THE FACE OF THE INDUSTRY A WOMAN MUST NOT ACCEPT; SHE MUST CHALLENGE. SHE MUST NOT BE AWED BY THAT WHICH HAS BEEN BUILT UP AROUND HER; SHE MUST REVERENCE THAT WOMAN IN HER WHICH STRUGGLES FOR EXPRESSION. Margaret Sanger Vision A just society where gender equality is a OUR reality VISION Mission Core To empower Purpose women and CORPOR ATE Advocacy for influence legisla- OUR VALUES gender equality tion and policy MISSION and equity for gender equal- ity in Uganda ACFODE Board of Directors 1. Dr. Euzobia Mugisha Baine - Chairperson 2. Jean Kemitare - Vice Chairperson Editor In Chief 3. Gladys Nairuba - Treasurer Contributors Sandra Nassali, [email protected] 4. Richard Makumbi 1. Janet Namayengo 5. Susan Bakesha 2. Brian Mutebi Editorial Team 6. Stedia Asiimwe 3. Owen Wagabaya 1. Regina Bafaki 7. Matilda Makata 4. Belinda Kyomuhendo 2. Hellen Twongyeirwe 8. Regina Bafaki - Secretary 5. Sasha Mumbi 3. Julius Ocwinyo 9. Sandra Nassali - Staff Representative 6. Stacey Pearl Keirungi ARISE 66 • 3 WE NEED WOMEN AT ALL LEVELS, INCLUDING THE TOP, TO CHANGE THE DYNAMIC, RESHAPE THE CONVERSATION, TO MAKE SURE WOMEN’S VOICES ARE HEARD AND HEEDED, NOT OVERLOOKED -

Stable Clients – Adults on ART for More Than 12 Months, Virally Suppressed with No Concurrent Illness Or Co- Morbidity and Demonstrated Good Adherence
Controversies in guidelines: Pediatrician's perspective 8th International Workshop on Women &HIV From Adolescence to Menopause BOSTON,MA,USA 2-3 March 2018 SABRINA BAKEERA-KITAKA,MD [email protected] Department of Paediatrics , Makerere University, Kampala , Uganda Faculty disclosures • No disclosures, and no conflict of interest • Travel grant from Workshop organizers Outline • ART Guidelines • Uganda Guidelines • Key issues for Pediatrics • Possible solutions Evolution of WHO ART Guidelines 90-90-90 and beyond 90 90% of people with HIV diagnosed • Novel point-of-care tools for early infant diagnosis of HIV 90% of diagnosed people treated • Guidelines for the managing advanced HIV disease and rapid initiation of antiretroviral therapy • Guidelines for the managing advanced HIV disease and rapid initiation of antiretroviral therapy • Transition to new antiretrovirals in HIV programmes • Transition to new antiretrovirals in HIV programmes • Key considerations for differentiated antiretroviral therapy delivery for specific populations: children, adolescents, pregnant and breastfeeding women and key populations 90% of people on treatment virally suppressed • Guidelines on the public health response to pretreatment HIV drug resistance • Tackling HIV drug resistance: trends, guidelines and global action Beyond 90-90-90 • WHO implementation tool for pre-exposure prophylaxis (PrEP) of HIV infection • Preventing HIV during pregnancy and breastfeeding in the context of PrEP WHO 2017 policy briefs The WHO HIV Guidelines are structured along the continuum of care Initiation of therapy CTX prophylaxis 1st L, 2nd L, 3rd L Cardiovascular HIV Diagnosis in Infant prophylaxis disease infants and children Depression PrEP TB diagnosis PEP Chronic Prevention Testing Link to care Treatment care Service delivery Children always lag behind despite the changes PMTCT Guidelines • Preventing MTCT has long been known as a highly effective intervention with huge potential to stem the tide of new pediatric HIV infections and improve both maternal and child health. -

National Antiretroviral Treatment and Care Guidelines for Adults, Adolescents, and Children
National Antiretroviral Treatment and Care Guidelines for Adults, Adolescents, and Children Ministry of Health Edited by: Elly T. Katabira, FRCP Moses R. Kamya, M. Med, MPH Israel Kalyesubula, M. Med Alice Namale, M. Med, DPH 2nd Edition – January 2008 Errors and omissions excepted. Every effort has been made to ensure that drug dosages and treatment schedules are correct and in accordance with current medical practice. However, medical knowledge is constantly and rapidly changing, particularly in relation to HIV/AIDS. Thus, when using an unfamiliar drug, clinicians are urged to confirm that information (especially with regards to drug usage) complies with the latest standards of practice. Hence these guidelines will need regular updating based on new knowledge, experiences and practices. We would welcome feedback and comments from the users and experts addressed to: The Director General Health Services Attn: Programme Manager STD/AIDS Control Programme Ministry of Health P.O. Box 7272 Kampala, Uganda ii Table of Contents TABLE OF CONTENTS...........................................................................................................................III ACRONYMS AND ABBREVIATIONS: ...................................................................................................V FOREWORD............................................................................................................................................ VII ACKNOWLEDGEMENTS ....................................................................................................................VIII -

Biobook YEAR 7 • 2018-2019 Orientation and Training July 15-20, 2018
FOGARTY GLOBAL HEALTH PROGRAM FOR FELLOWS AND SCHOLARS BioBook YEAR 7 • 2018-2019 Orientation and Training July 15-20, 2018 The Global Health Program for Fellows and Scholars* provides supportive mentorship, research opportunities and a collaborative research environment for early stage investigators from the U.S. and low- and middle-income countries (LMICs), as defined by the World Bank, to enhance their global health research expertise and their ca- reers. Six Consortia (funded in part by the Fogarty International Center [FIC] through competitive grants) identify postdoctoral Fellows and doctoral Scholars: Global Health Equity Scholars (GHES) University of California, Berkeley Florida International University Stanford University Yale University University of California Global Health Institute (UCGHI) GloCal Health Fellowship Program UC San Francisco UC San Diego UC Los Angeles UC Davis The HBNU Fogarty Global Health Fellowship Program (HBNU) Harvard University Northwestern University Boston University University of New Mexico The Northern Pacific Global Health Research Fellows Training Consortium (NPGH) University of Washington University of Hawaii University of Michigan University of Minnesota The UJMT Fogarty Global Health Fellowship Consortium (UJMT) The University of North Carolina-Chapel Hill Johns Hopkins University Morehouse School of Medicine Tulane University The VECD Global Health Fellowship Consortium (VECD) Vanderbilt University Emory University Cornell University The following NIH Institutes, Centers and Offices are collaborating -

Here It Was Called the Locations
4M+: PERINATAL PEER MENTORING PROGRAMME FOR WOMEN LIVING WITH HIV 4M STANDS FOR MY HEALTH, MY CHOICE, MY CHILD, MY LIFE Advocacy Brief about the 4M+ Programme Handmade book filled with personal advice and coping strategies BACKGROUND During 2017, Salamander Trust, in reproductive health and rights and, in so collaboration with PIPE Kenya and doing, to reduce chances of vertical UNYPA Uganda, implemented a Peri- transmission. The programme also Natal Peer Mentoring programme with focused on skilling the young mothers to young women living with HIV, with support their peers in similar ways funding from the MAC AIDS Foundation. through their pregnancy journeys. The programme provides safe spaces for The programme aimed to empower women to share, connect and support young women with knowledge on safe each other. motherhood and HIV, primarily to uphold the young women’s own sexual and 4M+ Page 2 The 4M+ programme was led by Twenty four of these mothers were Salamander Trust Associate Angelina below 24 years. The training built a Namiba, who has herself gone through team of ‘Mentor Mothers’ who the pregnancy journey whilst living voluntarily supported their peers in the with HIV. She originally created this community and health facilities. The programme in the UK with Positively training lasted 3 days in each of the 4 UK in London, where it was called the locations. It was highly interactive and “From Pregnancy to Baby and Beyond” covered several topics, including pre- programme. With Salamander Trust, conception planning, antenatal and the programme was then renamed 4M post-natal care, safe motherhood, and has spread to centres across the creative visioning, writing and drawing, UK. -
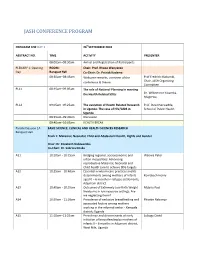
Conference Programme
JASH CONFERENCE PROGRAM PROGRAM FOR DAY 1 26thSEPTEMBER 2018 ABSTRACT NO. TIME ACTIVITY PRESENTER 08:00am–08:30am Arrival and Registration of Participants PLENARY 1: Opening ROOM: Chair: Prof. Rhoda Wanyenze Day Banquet Hall Co-Chair: Dr. Patrick Kadama 08:30am–08:45am Welcome remarks, overview of the Prof Fredrick Makumbi, conference & theme Chair, JASH Organizing Committee PL11 08:45am–09:05am The role of National Planning in meeting the Health Related SDGs Dr. Wilberforce Kisamba- Mugerwa, PL12 09:05am–09:25am The evolution of Health Related Research Prof. David Serwadda, in Uganda: The case of HIV/AIDS in School of Public Health Uganda 09:25am–09:40am Discussion 09:40am–10:05am HEALTH BREAK Parallel Session 1A BASIC SCIENCE, CLINICAL AND HEALTH SCIENCES RESEARCH Banquet Hall Track 1: Maternal, Neonatal, Child and Adolescent Health, Rights and Gender Chair: Dr. Elizabeth Nabiwemba Co-Chair: Dr. Sabrina Kitaka A11 10:10am - 10:25am Bridging regional, socioeconomic and Waiswa Peter urban inequalities: Advancing reproductive Maternal, Neonatal and Child health care to achieve SDG targets. A12 10:25am - 10:40am Essential newborn care practices and its determinants among mothers of infants Komakech Henry aged 0 – 6 months in refugee settlements, Adjumani district A13 10:40am - 10:55am Outcomes of Extremely Low Birth Weight Mubiru Paul Newborns in low resource settings: Are we neglecting them? A14 10:55am - 11:10am Prevalence of exclusive breastfeeding and Phoebe Nabunya associated factors among mothers working in the informal sector - Kampala district; Uganda A15 11:10am–11:25am Prevalence and determinants of early Lubogo David initiation of breastfeeding by mothers of infants 0 – 6 months in Adjumani district, West Nile, Uganda PROGRAM FOR DAY 1 26thSEPTEMBER 2018 ABSTRACT NO. -

Correlates of Ever Had Sex Among Perinatally HIV-Infected Adolescents in Uganda Scovia Nalugo Mbalinda1*, Noah Kiwanuka2, Lars E
Mbalinda et al. Reproductive Health (2015) 12:96 DOI 10.1186/s12978-015-0082-z RESEARCH Open Access Correlates of ever had sex among perinatally HIV-infected adolescents in Uganda Scovia Nalugo Mbalinda1*, Noah Kiwanuka2, Lars E. Eriksson3,4,5, Rhoda K. Wanyenze2 and Dan Kabonge Kaye6 Abstract Background: The objective of this study was to explore the correlates of ever had sex among perinatally HIV-infected (PHIV) adolescents. Methods: A cross-sectional survey of sexual behaviour was conducted with 624 PHIV adolescents living three regions (12 districts) of Uganda. Data was collected on socio demographic characteristics (age, sex, occupation, religion and education status), sexual practices and behaviours (Intimate relationships, sexual intercourse, age of sexual debut, condom use, multiple and concurrent sexual partners), consequences of sexual behaviours (pregnancy and STI’s) and life style factors (use of alcohol, psychoactive substances and peer influence). Multivariable logistic-regression was used to ascertain the determinants of sexual activity. Results: The majority of PHIV were female (59.3 %) and the mean age of the sample was 16.2 (±2.1) years. The mean age of sexual debut was 15.8 years; 16.2 % (101/624) reported symptoms for sexually transmitted infections (STI) and more than a third (213/624) reported ever had sex.Of these 76.5 % (165/213) used condoms inconsistently; and 49.3 % (105/213) had been pregnant or made someone pregnant. Of those in relationships, 56.3 % (223/396) did not disclose and were not aware of their partners’ HIV status. Adolescents aged 15–19 years were more likely to have ever been sexually active (Adjusted odds ratio (AOR) 6.28, 95 % Confidence interval (CI): 2.63-14.99) compared to those aged 10–14 years. -
The Women Human Rights Defenders Conference 2019
THE WOMEN HUMAN RIGHTS DEFENDERS CONFERENCE 2019 THEME: STEP INTO YOUR PURPOSE AND REFOCUS FOR 2020 DATE: 12TH DECEMBER, 2019 VENUE: GOLF COURSE HOTEL, KAMPALA The Human Rights Centre Uganda Copyright © 2020 All rights reserved. No part of this publication may be reproduced, stored in a retrieval system, or transmitted in any form or by any means without the prior permission of the publisher. Published by The Human Rights Centre, Uganda, Plot 198, Mugulusi Close, Kyanja P.O Box 25638, Kampala, Uganda Tel: +256 414 266186 / +256 704 424466 Toll Free: 0800333000 Email: [email protected] Website: www.hrcug.org Twitter: www.twitter.com/hrcug Facebook: www.facebook.com/hrcug THE WOMEN HUMAN RIGHTS DEFENDERS CONFERENCE 2019 Abbreviations and Acronyms. i. HRCU The Human Rights Centre Uganda ii. WHRD Women Human Rights Defender iii. PWG Phenomenal Women Uganda iv. ED Executive Director v. CEO Chief Executive Director vi. Phenomenal Women Global (PWG) vii. PWU Phenomenal Women Global viii. OIKOS ix. NGOs Non-Government Organizations x. IV Intravenous xi. ULS Uganda Law Society THE WOMEN HUMAN RIGHTS DEFENDERS CONFERENCE 2019 Background In October 2017, HRCU launched and economically empowered. its first thematic report on Women Human Right Defenders Basing on the above background, (WHRDs). Women human rights HRCU in 2018 organized inaugural defenders (WHRDs) are broadly Women HRD conference at Golf defined as both men and women Course hotel where Women HRDs who work in defense of women’s shared various issues they had rights or on gender issues. Many faced in the due course of their WHRDs work without realizing work. -

Makerere Medical Journal (MMJ), 48Th Edition
MMJ MMJ MAKEREREMEDICAL JOURNAL 48TH VOLUME chs.mak.ac.ug/studentjournals 1 5 31 51 CONTENTS CASE REPORTS 27 NEUROINTENSIVE MANAGEMENT OF ACUTE BACTERIAL MENINGITIS IN RESOURCE LIMITED 27 Foreword MMJ Editor in Chief / MUMSA president / Patron MMJ 3 SETTINGS MMJ Editors & Reviewers 4 NON-EDEMATOUS SEVERE ACUTE MALNUTRITION (SAM) COMPLICATED WITH 31 ACUTE WATERY DIARRHEA PERFORATED PEPTIC ULCER PRESENTING AFTER ORIGINAL RESEARCH 5 AN ACUTE SEVERE ILLNESS 37 KNOWLEDGE, ATTITUDES AND PRACTICES OF MAKERERE UNIVERSITY STUDENTS TOWARDS 5 VOLUNTARY BLOOD DONATION HEPATITIS B VACCINATION STATUS AMONG STUDENTS AT THE COLLEGE OF HEALTH SCIENCES, 15 PROJECT REPORTS 40 MAKERERE UNIVERSITY, UGANDA INCIDENCE OF CANCER AMONG CHILDREN AND THE HEARTSTRINGS’ PROJECT’ 40 ADOLESCENTS IN KYADONDO COUNTY, UGANDA 18 IMPROVING MENSTRUAL HYGIENE AMONG BETWEEN 2009 AND 2014 ADOLESCENTS IN FOUR SELECTED SCHOOLS IN 43 FORMULATION AND EVALUATION OF AN AMOLATAR DISTRICT ANTIDERMATOPHYTIC OINTMENT FROM 23 THE TOTO PROJECT: MAKING MATERNAL AND Eucalyptus Citriodora ESSENTIAL OIL CHILD HEALTH A COMMUNITY OBJECTIVE 46 EDITORIALS 50 DEPRESSION: SEEK HELP 49/53 POLLUTION CONTROL, A STRATEGY NOT TO FORGET! 50 THE EVOLUTION OF EMERGENCY MEDICINE AT MAKERERE UNIVERSITY 51 MEDICAL TRANSLATION: A NEGLECTED YET VITAL IN UGANDAN MEDICINE FIELD OFTEN 52 2 chs.mak.ac.ug/studentjournals Forewords MMJ elcome to the 48th Edition of the Makerere Medical Journal (MMJ). It is such a great milestone to which we shall always look back medical research and publication to renew medical practices Wand technologies in our academic and clinical lives. cutting edge research and publication is what we all call for. Therefore, as medical students, we need to build to it day by day starting at the smallest point of impact.