Actemra® (Tocilizumab) Injection for Intravenous Infusion
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Accuracy of Ontario Health Administrative Databases in Identifying Patients with Rheumatoid Arthritis
ACCURACY OF ONTARIO HEALTH ADMINISTRATIVE DATABASES IN IDENTIFYING PATIENTS WITH RHEUMATOID ARTHRITIS by Jessica Widdifield A thesis submitted in conformity with the requirements for the degree of Doctor of Philosophy in Health Services Research Institute of Health Policy, Management & Evaluation University of Toronto © Copyright by Jessica Widdifield 2013 Accuracy of Ontario Health Administrative Databases in Identifying Patients with Rheumatoid Arthritis (RA): Creation of the Ontario RA administrative Database (ORAD) Jessica Widdifield Doctor of Philosophy Institute of Health Policy Management and Evaluation University of Toronto 2013 Abstract Rheumatoid arthritis (RA) is a chronic, destructive, inflammatory arthritis that places significant burden on the individual and society. This thesis represents the most comprehensive effort to date to determine the accuracy of administrative data for detecting RA patients; and describes the development and validation of an administrative data algorithm to establish a province-wide RA database. Beginning with a systematic review to guide the conduct of this research, two independent, multicentre, retrospective chart abstraction studies were performed amongst two random samples of patients from rheumatology and primary care family physician practices, respectively. While a diagnosis by a rheumatologist remains the gold standard for establishing a RA diagnosis, the high prevalence of RA in rheumatology clinics can falsely elevate positive predictive values. It was therefore important we also perform a validation study in a primary care setting where prevalence of RA would more closely approximate that observed in the general population. The algorithm of [1 hospitalization RA code] OR [3 physician RA diagnosis codes (claims) with !1 by a specialist in a 2 year period)] demonstrated a ii high degree of accuracy in terms of minimizing both the number of false positives (moderately good PPV; 78%) and true negatives (high specificity: 100%). -

Rheumatoid Nodules Injected Area Six Times Daily; Nodules Differential Diagnosis and Immunological Appeared in 37 of 82 of Their Patients (450/O)
Matters arising 83 3 Paolaggi J B, Chaouat D, Auquier L. An rheumatic fever, though arthritis or arthralgia but my efforts to reproduce them additional test for the diagnosis and is of a more prolonged nature appearing early experimentally failed miserably! monitoring of giant cell arteritis and after infection. polymyalgia rheumatica. Arth Rheum 1985; F DUDLEY HART Ann Rheum Dis: first published as 10.1136/ard.53.1.83 on 1 January 1994. Downloaded from 28: 837-8. Massell et al' reported in 1937 that nodules 24 Harnnont House, closely resembling those of rheumatic fever 20 Harley Street, could be produced by injecting 3 cc of a London WIN IAN, patient's blood subcutaneously into the soft United Kingdom tissue of an elbow followed by rubbing the 1 Veys E M, De Keyser F. Rheuamtoid nodules: Rheumatoid nodules injected area six times daily; nodules differential diagnosis and immunological appeared in 37 of 82 of their patients (450/o). findings. Ann Rheum Dis 1993; 52: 625-6. 2 Schlesinger B E. The public health aspects of The recent Leader on rheumatoid nodules by I was unable to confirm these findings5 in 40 heart disease in children. Lancet 1938; 1: Veys and De Keyser' gave a very interesting children with rheumatic fever in acute or 593-9, 649-54. and useful review of the subject. When I was convalescent stages, five children with 3 Fink C W. The role of the streptococcus in post- registrar with Dr chronic juvenile arthritis (Still's disease), and streptococcal reactive arthritis in childhood working as a medical polyarteritis nodosa. -

Cancer Drug Pharmacology Table
CANCER DRUG PHARMACOLOGY TABLE Cytotoxic Chemotherapy Drugs are classified according to the BC Cancer Drug Manual Monographs, unless otherwise specified (see asterisks). Subclassifications are in brackets where applicable. Alkylating Agents have reactive groups (usually alkyl) that attach to Antimetabolites are structural analogues of naturally occurring molecules DNA or RNA, leading to interruption in synthesis of DNA, RNA, or required for DNA and RNA synthesis. When substituted for the natural body proteins. substances, they disrupt DNA and RNA synthesis. bendamustine (nitrogen mustard) azacitidine (pyrimidine analogue) busulfan (alkyl sulfonate) capecitabine (pyrimidine analogue) carboplatin (platinum) cladribine (adenosine analogue) carmustine (nitrosurea) cytarabine (pyrimidine analogue) chlorambucil (nitrogen mustard) fludarabine (purine analogue) cisplatin (platinum) fluorouracil (pyrimidine analogue) cyclophosphamide (nitrogen mustard) gemcitabine (pyrimidine analogue) dacarbazine (triazine) mercaptopurine (purine analogue) estramustine (nitrogen mustard with 17-beta-estradiol) methotrexate (folate analogue) hydroxyurea pralatrexate (folate analogue) ifosfamide (nitrogen mustard) pemetrexed (folate analogue) lomustine (nitrosurea) pentostatin (purine analogue) mechlorethamine (nitrogen mustard) raltitrexed (folate analogue) melphalan (nitrogen mustard) thioguanine (purine analogue) oxaliplatin (platinum) trifluridine-tipiracil (pyrimidine analogue/thymidine phosphorylase procarbazine (triazine) inhibitor) -

In Vivo Characterization of Combination Antitumor Chemotherapy with Calcium Channel Blockers and Ci5-Diamminedichloroplatinum(II)1
(CANCER RESEARCH 49, 2844-2850. June 1, 1989] In Vivo Characterization of Combination Antitumor Chemotherapy with Calcium Channel Blockers and ci5-Diamminedichloroplatinum(II)1 James M. Onoda,2 Kevin K. Nelson, John D. Taylor, and Kenneth V. Honn Departments of Radiation Oncology [J. M. O., K. K. N., J. D. T., K. V. H.], Biological Sciences [J. D. T.], and Chemistry ¡K.V. H.], Wayne State University, Detroit, Michigan 48202; and the Cershenson Radiation Oncology Center [J. M. O., K. K. N., J. D. T., K. V. H.], Harper/Grace Hospitals, Detroit, Michigan 4820I ABSTRACT dulin antagonists to enhance the antitumor actions of the more We have examined nifedipine, a dihydropyridine class calcium channel commonly prescribed organic or natural product chemothera blocker, for ability to overcome m-diamminedichloroplatinum(II) (cis- peutic agents (3, 4). The ability of verapamil to reverse multi- platin) resistance in a murine tumor line variant, B16a-Pt, which we drug resistance or pleiotropic drug resistance correlates with developed for resistance to cisplatin. Nifedipine significantly enhanced the expression of a M, 170,000 glycoprotein in drug-resistant the antitumor actions of cisplatin against primary subcutaneous B16a-Pt tumor cell plasma membranes (5, 6). This glycoprotein is now tumors and their spontaneous pulmonary métastases.We have charac commonly referred to as the P-glycoprotein (7, 8) and is re terized, in vivo, the pharmacokinetics and dose-response interactions sponsible for the active efflux of many organic/natural product between nifedipine and cisplatin. We now report our studies designed to cytotoxic chemotherapeutic agents (9-11). The current hypoth compare, in vivo, the efficacy of nifedipine and other calcium active esis suggests that verapamil interacts with the P-glycoprotein compounds including: (a) structurally similar calcium channel blockers to block drug efflux (12, 13); and that its actions are independ (nimodipine, nicardipine) from the dihydropyridine class, (b) structurally ent of the classical slow-inward Ca2+ channel (14, 15). -

Rheumatoid Nodules Developing Under Methotrexate Treatment for Rheumatoid Arthritis
Letters to the Editor 397 Rheumatoid Nodules Developing under Methotrexate Treatment for Rheumatoid Arthritis Sir, several punched-out radiolucencies and non-calcifying nodules Rheumatoid nodules have been reported to occur in about projecting in the soft parts surrounding the joints. 20% of patients with chronic rheumatoid polyarthritis (1, 2). In the recent literature, a correlation between treatment of rheumatoid arthritis with methotrexate and the development DISCUSSION or worsening of rheumatoid nodules has been suggested (2 ± 4). Methotrexate, a common drug for the treatment of rheuma- We describe here the explosive deterioration of rheumatoid toid arthritis, has been used successfully, but various side- nodules during the course of methotrexate treatment for effects have been reported. Among patients with rheumatoid rheumatoid polyarthritis in a 63-year-old woman and discuss arthritis under methotrexate therapy, the ones whose joint current aspects of possible pathogenetic mechanisms as well symptoms are improving due to administration of metho- as therapeutic options for methotrexate-induced rheumatoid trexate frequently develop subcutaneous nodules increasing in nodules. size and number (1 ± 3, 5). Nevertheless, only in rare cases the severity or the localization of the nodules, e.g. vascular, pulmonary or cardiac manifestation, necessitated discontinua- CASE REPORT tion of the drug (3). Cause and effect relationship between methotrexate treatment and the accelerated development of A 63-year-old woman presented to our department with a 15-year nodules is supported by the regression of the nodules after history of chronic rheumatoid polyarthritis affecting her ®ngers, discontinuation of the drug (2, 6). Although the predominant hand, elbow and knee joints. Ten years previously, she ®rst developed a few ®rm nodules on her ®ngers and feet. -

The Immunosuppressive Effects of Long-Term Combination Chemotherapy in Children with Acute Leukemia Inremission1
[CANCER RESEARCH 31, 420-426, April 1971] The Immunosuppressive Effects of Long-Term Combination Chemotherapy in Children with Acute Leukemia in Remission1 Luis Borella and Robert G. Webster Laboratories of Virology and Immunology, St. Jude Children 'sResearch Hospital, Memphis, Tennessee 38101 SUMMARY effects of prolonged maintained combination chemotherapy upon immunocompetence have not been investigated. The immunosuppressive effects of maintenance Knowledge of the immunosuppressive effects of long-term combination chemotherapy given for periods ranging from 8 combination chemotherapy is now urgently needed because of to 28 months to 20 children with acute lymphocytic leukemia the increasing number of prolonged remissions and potential in remission were investigated. There was depression of both 5-year cures among children with acute lymphocytic leukemia the primary antibody production to the hemagglutinin antigen receiving this form of treatment (19). of the Hong Kong influenza virus and the anamnestic response This study was aimed at determining the to the neuraminidase of the same virus. The primary response immunosuppressive effects of long-term combination (hemagglutination inhibition) was affected to a greater extent chemotherapy in children with acute lymphocytic leukemia. than the secondary (neuraminidase inhibition) response. A Several unique features of this investigation were as follows. preferential depression of 2-mercaptoethanol-resistant (a) All patients were in remission and received the same hemagglutination inhibition antibodies (IgG) was also combination chemotherapy continuously for periods ranging observed. One-fourth of all acute lymphocytic leukemia from 8 to 28 months, (b) Patients and controls were patients had low serum IgG levels. In vitro transformation of immunized with the Hong Kong influenza virus vaccine prior lymphocytes was a poor index of immunocompetence. -

Complicated Rheumatoid Nodules in Lung
Hindawi Case Reports in Rheumatology Volume 2020, Article ID 6627244, 3 pages https://doi.org/10.1155/2020/6627244 Case Report Complicated Rheumatoid Nodules in Lung Geetha Wickrematilake Sirimavo Bandaranayake Specialized Childrens Hospital, Kandy, Sri Lanka Correspondence should be addressed to Geetha Wickrematilake; [email protected] Received 18 October 2020; Revised 20 November 2020; Accepted 24 November 2020; Published 3 December 2020 Academic Editor: Gregory J. Tsay Copyright © 2020 Geetha Wickrematilake. ,is is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. A 65-year-old nonsmoker lady carrying a diagnosis of seropositive erosive rheumatoid arthritis for nine years presented with acute shortness of breath, following a spontaneous pneumothorax while on combination therapy with methotrexate, leflunomide, and tocilizumab. Imaging studies revealed multiple cavitory lung nodules, and a transbronchial lung biopsy favoured a diagnosis of rheumatoid lung nodules. Her initial pathological samples were negative for any infectious cause. A follow-up computerized tomography scan (CT scan) confirmed enlargement of lung nodules with a positive antibody test for aspergillosis which needed antifungal therapy, and currently, her arthritis is managed well with rituximab therapy, sulfasalazine, and hydroxychloroquine. 1. Introduction ,e oxygen saturation was 98% while breathing room air, but -

Actemra® (Tocilizumab) Injection for Intravenous Infusion
UnitedHealthcare® Community Plan Medical Benefit Drug Policy Actemra® (Tocilizumab) Injection for Intravenous Infusion Policy Number: CS2021D0043T Effective Date: September 1, 2021 Instructions for Use Table of Contents Page Commercial Policy Application ..................................................................................... 1 • Actemra® (Tocilizumab) Injection for Intravenous Coverage Rationale ....................................................................... 1 Infusion Applicable Codes .......................................................................... 4 Background.................................................................................. 14 Clinical Evidence ......................................................................... 14 U.S. Food and Drug Administration ........................................... 18 References ................................................................................... 18 Policy History/Revision Information ........................................... 19 Instructions for Use ..................................................................... 19 Application This Medical Benefit Drug Policy does not apply to the states listed below; refer to the state-specific policy/guideline, if noted: State Policy/Guideline Indiana Immunomodulators for Inflammatory Conditions (for Indiana Only) Kansas Refer to the state’s Medicaid clinical policy ® Kentucky Actemra (Tocilizumab) Injection for Intravenous Infusion (for Kentucky Only) Louisiana Refer to the state’s Medicaid clinical -

Non-Bacterial Thrombotic Endocarditis in a Patient With
Case Report http://dx.doi.org/10.4070/kcj.2016.46.3.425 Print ISSN 1738-5520 • On-line ISSN 1738-5555 Korean Circulation Journal Non-Bacterial Thrombotic Endocarditis in a Patient with Rheumatoid Arthritis Jung-Hye Choi, MD1, Jeong-Eun Park, MD1, Jang-Young Kim, MD2, and Taeyoung Kang, MD1 1Department of Rheumatology, 2Department of Cardiology, Yonsei University Wonju College of Medicine, Wonju, Korea Rheumatoid arthritis (RA) is frequently associated with various extra-joint complications. Although rare, thromboembolic complications are associated with high morbidity and mortality. We experienced a very rare case of nonbacterial thrombotic endocarditis (NBTE) and subsequent embolic stroke in a patient with RA. A 72-year-old male with a 15-year history of RA suddenly developed neurologic symptoms of vomiting and dizziness. Brain magnetic resonance imaging revealed recently developed multiple cerebellar and cerebral lacunar infarctions. Echocardiography showed a pulsating mitral valve vegetation involving the posterior cusp of the mitral valve leaflet, which was confirmed as NBTE. Immediate anti-coagulation therapy was started. The NBTE lesion disappeared in follow-up echocardiography after 4 weeks of anti-coagulation treatment. (Korean Circ J 2016;46(3):425-428) KEY WORDS: Endocarditis, non-infective; Arthritis, rheumatoid; Mitral valve. Introduction of life. Although RA patients have an increased risk of cardiovascular events such as myocardial infarction, stroke, cardiac death and Nonbacterial thrombotic endocarditis (NBTE), a very rare condition thromboembolism compared with the general population,2)3) there that refers to a spectrum of noninfectious endocarditis of the has been no report of mitral valvular NBTE in a patient with RA. -

SUPPLEMENTARY APPENDIX 4: Search Strategies Syntax Guide For
SUPPLEMENTARY APPENDIX 4: Search Strategies Pubmed, Embase Perioperative Management - PubMed Search Strategy – March 6, 2016 Syntax Guide for PubMed [MH] = Medical Subject Heading, also [TW] = Includes all words and numbers in known as MeSH the title, abstract, other abstract, MeSH terms, MeSH Subheadings, Publication Types, Substance Names, Personal Name as Subject, Corporate Author, Secondary Source, Comment/Correction Notes, and Other Terms - typically non-MeSH subject terms (keywords)…assigned by an organization other than NLM [SH] = a Medical Subject Heading [TIAB] = Includes words in the title and subheading, e.g. drug therapy abstracts [MH:NOEXP] = a command to retrieve the results of the Medical Subject Heading specified, but not narrower Medical Subject Heading terms Boolean Operators OR = retrieves results that include at least AND = retrieves results that include all the one of the search terms search terms NOT = excludes the retrieval of terms from the search Perioperative Management PubMed Search Strategy – March 6, 2016 Search Query #1 ((((ARTHROPLASTY, REPLACEMENT, HIP[MH] OR HIP PROSTHES*[TW] OR HIP REPLACEMENT*[TIAB] OR HIP ARTHROPLAST*[TIAB] OR HIP TOTAL REPLACEMENT*[TIAB] OR FEMORAL HEAD PROSTHES*[TIAB]) OR (ARTHROPLASTY, REPLACEMENT, KNEE[MH] OR KNEE PROSTHES*[TW] OR KNEE REPLACEMENT*[TW] OR KNEE ARTHROPLAST*[TW] OR KNEE TOTAL REPLACEMENT*[TIAB]) OR (ARTHROPLAST*[TW] AND (HIP[TIAB] OR HIPS[TIAB] OR KNEE*[TIAB])) AND (("1980/01/01"[PDAT] : "2016/03/06"[PDAT]) AND ENGLISH[LANG])) NOT (((("ADOLESCENT"[MESH]) OR "CHILD"[MESH]) -

Mtorc1/2 Inhibition Preserves Ovarian Function and Fertility During Genotoxic Chemotherapy
mTORC1/2 inhibition preserves ovarian function and fertility during genotoxic chemotherapy Kara N. Goldmana, Devon Chenetteb, Rezina Arjub, Francesca E. Duncanc, David L. Keefea, Jamie A. Grifoa, and Robert J. Schneiderb,d,1 aDivision of Reproductive Endocrinology and Infertility, Department of Obstetrics and Gynecology, New York University School of Medicine, New York,NY 10016; bDepartment of Microbiology, New York University School of Medicine, New York, NY 10016; cDepartment of Obstetrics and Gynecology, Feinberg School of Medicine, Northwestern University, Chicago, IL 60611; and dPerlmutter Cancer Center, New York University School of Medicine, New York, NY 10016 Edited by Nahum Sonenberg, McGill University, Montreal, QC, Canada, and approved February 8, 2017 (received for review October 17, 2016) The ovary contains oocytes within immature (primordial) follicles pathway, leading to primordial follicle activation and follicular that are fixed in number at birth. Activation of follicles within this “burnout” (6, 7). fixed pool causes an irreversible decline in reproductive capacity, Ovarian folliculogenesis initiates from the primordial follicle known as the ovarian reserve, until menopause. Premenopausal stage, where an oocyte arrested in prophase of meiosis I and women undergoing commonly used genotoxic (DNA-damaging) surrounded by a single layer of squamous granulosa cells is ac- chemotherapy experience an accelerated loss of the ovarian tivated to grow and transition to a primary follicle, secondary reserve, leading to subfertility and infertility. Therefore, there is follicle, and then ultimately a preovulatory antral follicle (Fig. considerable interest but little effective progress in preserving S1). Most oocytes within the ovary exist in a quiescent state ovarian function during chemotherapy. Here we show that block- within primordial follicles, relatively resistant to antimitotic and ing the kinase mammalian/mechanistic target of rapamycin genotoxic agents (8, 9). -
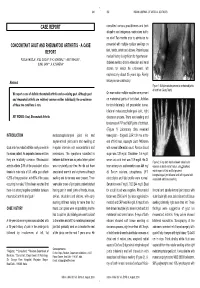
CONCOMITANT GOUT and RHEUMATOID ARTHRITIS - a CASE Presented with Multiple Nodular Swellings on REPORT Feet, Hands, Wrists and Elbows
349 350 INDIAN JOURNAL OF MEDICAL SCIENCES CASE REPORT consulted various practitioners and took allopathic and indigenous medications but to no relief. Two months prior to admission he CONCOMITANT GOUT AND RHEUMATOID ARTHRITIS - A CASE presented with multiple nodular swellings on REPORT feet, hands, wrists and elbows. Patient’s past medical history is significant for hypertension, POOJA KHOSLA*, ATUL GOGIA**, P. K. AGARWAL***, AMIT PAHUJA**, SUNIL JAIN***, K. K. SAXENA# diabetes mellitus, chronic ethanolism and renal stones for which he underwent left nephrectomy about 25 years ago. Family Abstract history is non-contributory. Figure 1: Multiple nodules present on metatarsal joints of both feet (Gouty Tophi) We report a case of definite rheumatoid arthritis and co-existing gout. Although gout On examination multiple nodules were present and rheumatoid arthritis are relatively common entities individually, the co-existence on metatarsal joints of both feet, Achilles of these two conditions is rare. tendon bilaterally, left prepatellar bursa, bilateral metacarpophalangeal joint, right KEY WORDS: Gout, Rheumatoid Arthritis olecranon process. There was swelling and tenderness of PIP and MCP joints of both feet. (Figure 1) Laboratory data revealed INTRODUCTION metatarsophalangeal joint. He had hemoglobin - 8.9gm/dl, ESR 124 mm at the asymmetrical joint pains and swelling at end of first hour, leucocyte count 7400/cmm Gout and rheumatoid arthritis rarely co-exist in irregular intervals with exacerbations and with normal differential count. Random blood the same patient. As separate disease entities remissions. The symptoms subsided in sugar was 139 mg/dl, Creatinine- 1.6 mg/dl; they are relatively common. Rheumatoid between but there was no period when patient serum uric acid level was 10.9 mg/dl.