1. Hydrogen Fundamentals
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Problems of Metallic Hydrogen and Room Temperature Superconductivity
EHPRG 2019 - Abstracts Problems of metallic hydrogen and room temperature superconductivity M.I. Eremets Max-Planck-Institut fur Chemie, Hahn-Meitner Weg 1, 55128 Mainz, Germany Keywords: high pressure, mrtallic hydrogen, room temperature superconductivity. *e-mail: [email protected] Metallic Hydrogen and Room-temperature hydrides at high pressures. We will present recent studies superconductivity (RTSC) are one the most challenging on YHx, CaHx, MgHx- other compounds which are and very long standing problems in solid-state physics. In considered as potential RTSCs. both, there is a significant progress over the recent years. We will consider various directions to explore high temperature conventional superconductivity at low and In 1935, Wigner and Huntington [1] predicted that ambient pressures. solid molecular hydrogen would dissociate at high pressure to form a metallic atomic solid at pressures Acknowledgments: This work was supported by the Max P370-500 GPa [1-3]. Besides the ultimate simplicity, Planck Society. atomic metallic hydrogen is attractive because of the 1. Wigner, E. and H.B. Huntington, On the possibility of predicted very high, room temperature for a metallic modification of hydrogen. J. Chem. Phys., 1935. 3: p. 764-770. superconductivity [4]. In another scenario, the 2. Pickard, C.J. and R.J. Needs, Structure of phase III of metallization first occurs in the 250-500 GPa pressure solid hydrogen. Nature Physics, 2007. 3. range in molecular hydrogen through overlapping of 3. McMinis, J., et al., Molecular to Atomic Phase electronic bands [5-8]. The calculations are not accurate Transition in Hydrogen under High Pressure. Phys. enough to predict which option is realized. -

Will Solid Hydrogen Ever Be a Metal?
news and views tures. The consequences of squeezing solid hydrogen are clearly a good deal more subtle Will solid hydrogen than at first thought, and it turns out that this, the ‘simplest’ of all molecular solids, has a rich and remarkably complex phase ever be a metal? diagram2,4,5 (Fig. 2). In phase I of solid hydrogen, the so-called orientationally disordered state, the individ- Peter P. Edwards and Friedrich Hensel ual molecules execute complete rotational At ultra-high pressures, liquid hydrogen becomes metallic. So should motion in addition to the usual molecular solid hydrogen, yet it stubbornly resists. A newly predicted vibrations. spontaneous asymmetry of molecules in the solid may be the reason. Below a temperature of about 120 K, solid hydrogen undergoes a transition at about n 1926 J. D. Bernal proposed that all The pressure-induced metallization of 1.5 million atmospheres to phase II, a state matter, when subjected to a high enough solid hydrogen can be viewed in terms of in which the constituent H2 molecules Ipressure, will inevitably become metallic electronic band theory. At low pressures the become ‘frozen’ in a random orientation in — that is, it will be permeated by a sea of solid is an infinite crystalline assembly of iso- the crystal. completely free electrons that conduct elec- lated H2 molecules, all weakly interacting. Phase III is undoubtedly the most tricity easily. The most enticing substance for The electrons are all bound to their mole- intriguing, because elemental hydrogen pressure-induced metallization is, in fact, cules, and need to be freed to conduct elec- remains in this state up to the highest pres- the lightest and supposedly the simplest of all tricity: in band-theory terms, we have a com- sures yet achieved, and still does not become the elements in the periodic table — hydro- pletely filled valence band separated by a very metallic. -

Saturn — from the Outside In
Saturn — From the Outside In Saturn — From the Outside In Questions, Answers, and Cool Things to Think About Discovering Saturn:The Real Lord of the Rings Saturn — From the Outside In Although no one has ever traveled ing from Saturn’s interior. As gases in from Saturn’s atmosphere to its core, Saturn’s interior warm up, they rise scientists do have an understanding until they reach a level where the tem- of what’s there, based on their knowl- perature is cold enough to freeze them edge of natural forces, chemistry, and into particles of solid ice. Icy ammonia mathematical models. If you were able forms the outermost layer of clouds, to go deep into Saturn, here’s what you which look yellow because ammonia re- trapped in the ammonia ice particles, First, you would enter Saturn’s up- add shades of brown and other col- per atmosphere, which has super-fast ors to the clouds. Methane and water winds. In fact, winds near Saturn’s freeze at higher temperatures, so they equator (the fat middle) can reach turn to ice farther down, below the am- speeds of 1,100 miles per hour. That is monia clouds. Hydrogen and helium rise almost four times as fast as the fast- even higher than the ammonia without est hurricane winds on Earth! These freezing at all. They remain gases above winds get their energy from heat ris- the cloud tops. Saturn — From the Outside In Warm gases are continually rising in Earth’s Layers Saturn’s atmosphere, while icy particles are continually falling back down to the lower depths, where they warm up, turn to gas and rise again. -
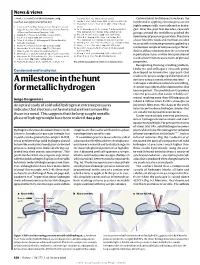
A Milestone in the Hunt for Metallic Hydrogen
News & views e-mails: [email protected]; Catterall, W. A. Sci. Signal. 3, ra70 (2010). Conventional techniques have been the [email protected] 11. Lemke, T. et al. J. Biol. Chem. 283, 34738–34744 (2008). bottleneck in applying extreme pressures to 12. Brandmayr, J. et al. J. Biol. Chem. 287, 22584–225924 1. Cannon, W. B. Bodily Changes in Pain, Hunger, Fear, and (2012). highly compressible materials such as hydro- Rage: An Account of Recent Researches into the Function 13. Fu, Y., Westenbroek, R. E., Scheuer, T. & Catterall, W. A. gen. Over the past few decades, research of Emotional Excitement (Appleton, 1915). Proc. Natl Acad. Sci. USA 110, 19621–19626 (2013). groups around the world have pushed the 2. Reuter, H. J. Physiol. (Lond.) 192, 479–492 (1967). 14. Rhee, H.-W. et al. Science 339, 1328–1331 (2013). 3. Liu, G. et al. Nature 577, 695–700 (2020). 15. Finlin, B. S., Crump, S. M., Satin, J. & Andres, D. A. boundaries of pressure generation. They have 4. Tsien, R. W., Giles, W. & Greengard, P. Nature New Biol. Proc. Natl Acad. Sci. USA 100, 14469–14474 (2003). also refined the tools and methods needed 240, 181–183 (1972). 16. Manning, J. R. et al. J. Am. Heart Assoc. 2, e000459 (2013). to accurately estimate pressures applied to a 5. Reuter, H. J. Physiol. (Lond.) 242, 429–451 (1974). 17. Yang, L. et al. J. Clin. Invest. 129, 647–658 (2019). 6. Osterrieder, W. et al. Nature 298, 576–578 (1982). 18. Bean, B. P., Nowycky, M. C. & Tsien, R. -

Liquid Metallic Hydrogen and the Structure of Brown Dwarfs and Giant Planets W.B
CORE Metadata, citation and similar papers at core.ac.uk Provided by CERN Document Server Liquid metallic hydrogen and the structure of brown dwarfs and giant planets W.B. Hubbard, T. Guillot, J.I. Lunine Lunar and Planetary Laboratory, University of Arizona, Tucson, AZ 85721 [email protected] A. Burrows Departments of Physics and Astronomy, University of Arizona, Tucson, AZ 85721 D. Saumon Department of Physics and Astronomy, Vanderbilt University, Nashville, TN 37235 M.S. Marley Department of Astronomy, New Mexico State University, Las Cruces, NM 88003 R.S. Freedman Sterling Software, NASA Ames Research Center, Moffett Field, CA 94035 ABSTRACT Electron-degenerate, pressure-ionized hydrogen (usually referred to as metallic hydrogen) is the principal constituent of brown dwarfs, the long-sought objects which lie in the mass range between the lowest-mass stars (about eighty times the mass of Jupiter) and the giant planets. The thermodynamics and transport properties of metallic hydrogen are important for understanding the properties of these objects, which, unlike stars, continually and slowly cool from initial nondegenerate (gaseous) states. Within the last year, a brown dwarf (Gliese 229 B) has been detected and its spectrum observed and analyzed, and several examples of extrasolar giant planets have been discovered. The brown dwarf appears to have a mass of about forty to fifty Jupiter masses and is now too cool to be fusing hydrogen or deuterium, although we predict that it will have consumed all of its primordial deuterium. This paper reviews the current understanding of the interrelationship between its interior properties and its observed spectrum, and also discusses the current status of research on the structure of giant planets, both in our solar system and elsewhere. -

Alamos Scientific Laboratory of the University of California 10S ALAMOS, NEW MEXICO A7545
LA-61 72-MS Informal Report UC-34a Reporting Date: November 1975 Issued: January 1976 CIC-14 REPORT COLLECTION REPRODUCTION COPY Metallic Hydrogen: A Brief Technical Assessment by L. A. Gritzo N. H. Krikorian —L Contributors D. T. Vier A. T. Peaslee, Jr. r ) } alamos scientific laboratory of the university of California 10s ALAMOS, NEW MEXICO a7545 \ An Affirmative Action/Equal Opportunity Employer uNITEO STATES ENERGY RESEARCH AND DEVELOPMENT ADMINISTRATION CONTRACT W.7405.ENG. 36 In the interest of prompt distribution, this report was not edited by the Technical Information staff. This work was supported by the DoD Defense Advanced Research Projects Agency. The views and conclusions contained in this report are those of the authors and should not be interpreted as necessarily representing the official policies, either expressed or implied, of the Defense Advanced Research Pro- jects Agency or of the United States Government. Printed in the United States of America. Available from Nationsl Technical Information Service U.S. Department of Commerce 6286 Port Royal Road Springfield. VA 22161 Price: Printed CopY $4.50 Microfiche $2.26 7%1, ,ePort w., Prep. red ,, . ●ccount ofwork ,p.a.,.,ed h k UnitedSW- Government. Neither the UqiwJSm- .or the U“itrd Stat” l?nersy Scwarch ..d Development Ad- ministration, nor any or their ●mpl.yccs. “or any of their con. tractors. ..bco.tr.clors, or their empl.>~, !n.kes .ny wmr..tv. ●xpress or implied, . ..s. nms .ny Irr.1 Ii.btlity or rcspywibility for the ●ccuracy, campleten”., or .sef.lnts. of an? mformattom apparatus, LWXIuct, w D,,JWSJ di~lo,~, or rrr.ramt. -

Saturn a Featured Article from Wikipedia, the Free Encyclopedia
Saturn A featured article from Wikipedia, the free encyclopedia This article is about the planet. For other uses, see Saturn (disambiguation). Saturn is the sixth planet from the Sun and the second-largest in the Solar System, after Jupiter. It is a gas giant with an average radius about nine times that of Earth.[10][11] Although only one-eighth the average density of Earth, with its larger volume Saturn is just over 95 times more massive.[12][13][14] Saturn is named after the Ro- man god of agriculture; its astronomical symbol () represents the god's sickle. Saturn's interior is probably composed of a core of iron–nickel and rock (silicon and oxygen com- pounds). This core is surrounded by a deep layer of Saturn in natural color, photographed by Cassini in metallic hydrogen, an intermediate layer of liquid July 2008, approaching equinox. hydrogen and liquid helium, and finally outside the Frenkel line a gaseous outer layer.[15] Saturn has a pale yellow hue due to ammonia crystals in its upper atmosphere. Electrical current within the metallic hydrogen layer is thought to give rise to Sat- urn's planetary magnetic field, which is weaker than Earth's, but has a magnetic moment 580 times that of Earth due to Saturn's larger size. Saturn's magnetic field strength is around one-twentieth of Jupiter's.[16] The outer atmosphere is generally bland and lacking in contrast, although long-lived fea- tures can appear. Wind speeds on Saturn can reach 1,800 km/h (500 m/s), higher than on Jupiter, but not as high as those on Neptune.[17] Saturn has a prominent ring system that consists of nine continuous main rings and three discontin- uous arcs and that is composed mostly of ice particles with a smaller amount of rocky debris and dust. -

High Pressure Metallic Hydrogen
Metallic Hydrogen Isaac F. Silvera Lyman Laboratory of Physics, Harvard University The SSAA program Jing Song, Albert Derrick VanGennep Azza Elobeid Kiran Linsuain, postdoc postdoc grad student undergrad; missing pic. Former Students now at the National Labs or SSAA support Will Evans Livermore Hector Lorenzana Livermore Jon Eggert Livermore Mohamed Zaghoo Laser Energetics-Omega, Rochester Todd Ditmire U. of Texas, Austin Former Postdocs Training the next generation in High Density research Ranga Dias U. of Rochester Shanti Deemyad U. of Utah Ashkan Salamat UNLV We pressurize and study hydrogen in a Diamond Anvil Cell (DAC) at low temperatures E. Wigner and H. B. Huntington, On the Possibility of a Metallic Modification of Hydrogen, J. Chem. Phys. 3, 764 (1935). 1935 Prediction: 25 GPa in the zero Temp. Limit Liquid? High Tc High Tc Supercond. Superconductivity Metastable Metal? Density: 1 13-14 ~15 Pressure 0 400-450 GPa? 400-500 GPa? Hexagonal Close Packed Structure of Para-Molecular Hydrogen at Low Pressure and Temp.--LP or Phase I The putative phase diagram of hydrogen showing Pathway I and II to the metallic phases. We have observed both transitions: • I The Wigner Huntington transition • II The liquid-liquid or PP Transition to liquid atomic hydrogen Our current project is to further understand Pathway I, but I shall start out with Pathway II as we have published results on on this last year. When we started working on the liquid liquid transition to liquid atomic hydrogen, the only experimental measurement was by Weir et al (S. T. Weir, A. C. Mitchell, and W. -
![Arxiv:2103.16282V2 [Cond-Mat.Supr-Con] 28 May 2021 Form to Design High Superconducting Hydrides](https://docslib.b-cdn.net/cover/3507/arxiv-2103-16282v2-cond-mat-supr-con-28-may-2021-form-to-design-high-superconducting-hydrides-1543507.webp)
Arxiv:2103.16282V2 [Cond-Mat.Supr-Con] 28 May 2021 Form to Design High Superconducting Hydrides
Experimental observation of superconductivity at 215 K in calcium superhydride under high pressures 1, 2, 3, 1, 2, 1, 2, 1, 2 1, 2 Liang Ma, ∗ Kui Wang, ∗ Yu Xie, ∗ Xin Yang, Yingying Wang, Mi Zhou,2 Hanyu Liu,1, 2 Hongbo Wang,1, 2, † Guangtao Liu,2, ‡ and Yanming Ma1, 2, 3, § 1State Key Laboratory of Superhard Materials, College of Physics, Jilin University, Changchun 130012, China 2International Center of Computational Method & Software, College of Physics, Jilin University, Changchun 130012, China 3International Center of Future Science, Jilin University, Changchun 130012, China (Dated: May 31, 2021) The flourishing metal clathrate superhydrides are a class of recently discovered materials that possess record breaking near-room-temperature superconductivity at high pressures, because hydrogen atoms behave similarly to the atomic metallic hydrogen. While series of rare-earth clathrate superhydrides have been realized, the superconductivity of the first proposed clathrate calcium superhydride that initiates this major discovery has not been observed yet and remains of fundamental interest in the field of high-pressure physics. Here, we report the synthesis of calcium superhydrides from calcium and ammonia borane precursors with a maximum superconducting temperature of 215 K at 172 GPa, confirmed by the observation of zero resistance through four-probe electrical transport measurements. An exceedingly high upper critical magnetic field was estimated to be 203 T at zero temperature in the Werthamer–Helfand–Hohenberg model. Inferred from the synchrotron X-ray diffraction, together with the consistencies of superconducting transition temperature and equation of states between experiment and theory, sodalite-like clathrate CaH6 is one of the best candidates for this high-Tc CaHx. -

Everything You Always Wanted to Know About Metallic Hydrogen but Were Afraid to Ask', Matter and Radiations at Extremes
Edinburgh Research Explorer Everything You Always Wanted to Know About Metallic Hydrogen but Were Afraid to Ask Citation for published version: Gregoryanz, E, Ji, C, Dalladay-Simpson, P, Li, B, Howie, RT & Mao, H-K 2020, 'Everything You Always Wanted to Know About Metallic Hydrogen but Were Afraid to Ask', Matter and Radiations at Extremes. https://doi.org/10.1063/5.0002104 Digital Object Identifier (DOI): 10.1063/5.0002104 Link: Link to publication record in Edinburgh Research Explorer Document Version: Peer reviewed version Published In: Matter and Radiations at Extremes General rights Copyright for the publications made accessible via the Edinburgh Research Explorer is retained by the author(s) and / or other copyright owners and it is a condition of accessing these publications that users recognise and abide by the legal requirements associated with these rights. Take down policy The University of Edinburgh has made every reasonable effort to ensure that Edinburgh Research Explorer content complies with UK legislation. If you believe that the public display of this file breaches copyright please contact [email protected] providing details, and we will remove access to the work immediately and investigate your claim. Download date: 20. Sep. 2020 1200 Molecular 1000 Fluid Metallic 800 Fluid 600 Temperature (K) Temperature 400 I IV V Metallic Solid 200 III II 0 100 200 300 400 500 Pressure (GPa) This is the author’s peer reviewed, accepted manuscript. However, the online version of record will be different from this version once it has been copyedited and typeset. PLEASE CITE THIS ARTICLE AS DOI:10.1063/5.0002104 Everything You Always Wanted to Know About Metallic Hydrogen but Were Afraid to Ask Eugene Gregoryanz1;2;3;∗, Cheng Ji2, Philip Dalladay-Simpson2, Bing Li2, Ross T. -

Semimetallic Molecular Hydrogen at Pressure Above 350 Gpa
Semimetallic molecular hydrogen at pressure above 350 GPa M. I. Eremets, A. P. Drozdov, P. P. Kong, H. Wang Max-Planck-Institut fur Chemie, Hahn-Meitner Weg 1, 55128 Mainz, Germany According to the theoretical predictions, insulating molecular hydrogen dissociates and transforms to an atomic metal at pressures P370-500 GPa1-3. In another scenario, the metallization first occurs in the 250-500 GPa pressure range in molecular hydrogen through overlapping of electronic bands4-7.The calculations are not accurate enough to predict which option is realized. Here we show that at a pressure of 360 GPa and temperatures <200 K the hydrogen starts to conduct, and that temperature dependence of the electrical conductivity is typical of a semimetal. The conductivity, measured up to 440 GPa, increases strongly with pressure. Raman spectra, measured up to 480 GPa, indicate that hydrogen remains a molecular solid at pressures up to 440 GPa, while at higher pressures the Raman signal vanishes, likely indicating further transformation to a good molecular metal or to an atomic state. Achieving a metallic state of hydrogen, predicted to occur at high pressure, is one of the most attractive goals in condense matter physics and remains a long-standing challenge both for theory and experiment. In 1935 Wigner and Huntington1 proposed that any lattice built of hydrogen atoms (protons) should display metallic properties similar to the alkali metals. However, a metallic state can be stabilized only at very high pressures 370-500 GPa 2,3,8. Besides the ultimate simplicity, atomic metallic hydrogen is attractive because of the predicted very high critical temperature for superconductivity9. -

High Temperature Equation of State of Metallic Hydrogen V
ISSN 1063-7761, Journal of Experimental and Theoretical Physics, 2007, Vol. 104, No. 4, pp. 655–660. © Pleiades Publishing, Inc., 2007. Original Russian Text © V.T. Shvets, 2007, published in Zhurnal Éksperimental’noœ i Teoreticheskoœ Fiziki, 2007, Vol. 131, No. 4, pp. 743–749. STATISTICAL, NONLINEAR, AND SOFT MATTER PHYSICS High Temperature Equation of State of Metallic Hydrogen V. T. Shvets Odessa State Academy of Refrigeration, Odessa, 65026 Ukraine *e-mail: [email protected] Received May 5, 2006 Abstract—The equation of state of liquid metallic hydrogen is solved numerically. Investigations are carried out at temperatures from 3000 to 20000 K and densities from 0.2 to 3 mol/cm3, which correspond both to the experimental conditions under which metallic hydrogen is produced on earth and the conditions in the cores of giant planets of the solar system such as Jupiter and Saturn. It is assumed that hydrogen is in an atomic state and all its electrons are collectivized. Perturbation theory in the electron–proton interaction is applied to deter- mine the thermodynamic potentials of metallic hydrogen. The electron subsystem is considered in the random- phase approximation with regard to the exchange interaction and the correlation of electrons in the local-field approximation. The proton–proton interaction is taken into account in the hard-spheres approximation. The thermodynamic characteristics of metallic hydrogen are calculated with regard to the zero-, second-, and third- order perturbation theory terms. The third-order term proves to be rather essential at moderately high tempera- tures and densities, although it is much smaller than the second-order term.