Survey, Surveillance & Cultural Characteristics of Dry Root Rot Of
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Kanpur Dehat District, U.P
DISTRICT GROUND WATER BROCHURE KANPUR DEHAT DISTRICT, U.P. (AAP: 2012-13) By P.K. Tripathi Scientist 'C' CONTENTS Chapter Title Page No. KANPUR DEHAT DISTRICT AT A GLANCE ..................3 1.0 INTRODUCTION ..................6 2.0 RAINFALL & CLIMATE ..................7 3.0 GEOMORPHOLOGY & SOIL TYPE ..................7 4.0 GROUND WATER SCENARIO ..................8 4.1 Hydrogeology 4.2 Ground Water Resource 4.3 Ground Water Quality 4.4 Status of Ground Water Development 5.0 GROUND WATER MANAGEMENT STRATEGY ..................14 5.1 Ground Water Development 5.2 Water Conservation Structure & Artificial Recharge 6.0 GROUND WATER RELATED ISSUES AND PROBLEMS ..................15 7.0 RECOMMENDATIONS ..................16 PLATES: I. INDEX MAP OF KANPUR DEHAT DISTRICT, U.P. II. HYDROGEOMORPHOLOGICAL MAP OF KANPUR DEHAT DISTRICT, U.P. III. CANAL'S MAP OF KANPUR DEHAT, U.P. IV. FENCE DIAGRAM KANPUR DEHAT, U.P. V. DEPTH TO WATER LEVEL PREMONSOON 2012, KANPUR DEHAT DISTRICT, U.P. VI. DEPTH TO WATER LEVEL POSTMONSOON 2012, KANPUR DEHAT DISTRICT, U.P. VII. CATEGORIZATION OF BLOCKS (GROUND WATER RESOURCES /DRAFT), KANPUR DEHAT DISTRICT, U.P. VIII. ISOCON MAP AND POINT VALUES OF ARSENIC (PHREATIC AQUIFER), KANPUR DEHAT DISTRICT, U.P. APPENDIX: I. DETAILS OF EXPLORATORY TUBEWELLS IN KANPUR DEHAT DISTRICT, U.P. 2 KANPUR DEHAT DISTRICT AT GLANCE 1. GENERAL INFORMATION i. Geographical Area (Sq km.) : 3021 ii. Administrative Divisions : Number of Tehsil/Block 5/10 Number of Panchayat/Villages 102/1032 iii. Population (as on 2001 census) : 15,63,336 iv. Average Annual Rainfall (mm) : 782.8 2. GEOMORPHOLOGY Major Physiographic Units : Older Alluvium plain, older flood plain & active flood plain Major Drainages : Yamuna, Pandu, Rind, Sengar 3. -

Studies on Adoption of Feeding Management Practices in District Kanpur Dehat (U.P.)
Int.J.Curr.Microbiol.App.Sci (2021) 10(08): 19-25 International Journal of Current Microbiology and Applied Sciences ISSN: 2319-7706 Volume 10 Number 08 (2021) Journal homepage: http://www.ijcmas.com Original Research Article https://doi.org/10.20546/ijcmas.2021.1008.003 Studies on Adoption of Feeding Management Practices in District Kanpur Dehat (U.P.) Narendra Kumar*, P. K. Upadhyay, Ramjee Gupta, M. P. S. Yadav, Deepak Singh and Satendra kumar Department of Animal Husbandry and Dairying, C. S. Azad University of Agriculture and Technology Kanpur - 208002, India *Corresponding author ABSTRACT The present study was carried out on feeding management practices on dairy animals, reared by 200 farmers’ viz: villages of the Maitha, Akbarpur, Derapur and Rasulabad blocks of Kanpur Dehat district. This is presented in central plane zone of Uttar Pradesh state. The result of the study revealed that among the total 1175 dairy animals maintained by the 200 dairy farmer maximum 57.10 per cent were buffaloes and 42.90 K e yw or ds per cent were cattle. Regarding feeding management practices in district there was 71.00 per cent of farmers grown the fodder crops. 77.50 per cent of farmer followed Feeding, Concentrate the stall feeding for animals and 85.00 per cent of farmers tried individual type of mixture, Green feeding for dairy animals. 55.50 per cent of farmers practicing of concentrate mixture Fodder, Dry fodder feeding to their animals on before milking. More than half farmers were fed the pattern of feeding as (GF+DF+Concentrate) for the animals. -

List of Common Service Centres Established in Uttar Pradesh
LIST OF COMMON SERVICE CENTRES ESTABLISHED IN UTTAR PRADESH S.No. VLE Name Contact Number Village Block District SCA 1 Aram singh 9458468112 Fathehabad Fathehabad Agra Vayam Tech. 2 Shiv Shankar Sharma 9528570704 Pentikhera Fathehabad Agra Vayam Tech. 3 Rajesh Singh 9058541589 Bhikanpur (Sarangpur) Fatehabad Agra Vayam Tech. 4 Ravindra Kumar Sharma 9758227711 Jarari (Rasoolpur) Fatehabad Agra Vayam Tech. 5 Satendra 9759965038 Bijoli Bah Agra Vayam Tech. 6 Mahesh Kumar 9412414296 Bara Khurd Akrabad Aligarh Vayam Tech. 7 Mohit Kumar Sharma 9410692572 Pali Mukimpur Bijoli Aligarh Vayam Tech. 8 Rakesh Kumur 9917177296 Pilkhunu Bijoli Aligarh Vayam Tech. 9 Vijay Pal Singh 9410256553 Quarsi Lodha Aligarh Vayam Tech. 10 Prasann Kumar 9759979754 Jirauli Dhoomsingh Atruli Aligarh Vayam Tech. 11 Rajkumar 9758978036 Kaliyanpur Rani Atruli Aligarh Vayam Tech. 12 Ravisankar 8006529997 Nagar Atruli Aligarh Vayam Tech. 13 Ajitendra Vijay 9917273495 Mahamudpur Jamalpur Dhanipur Aligarh Vayam Tech. 14 Divya Sharma 7830346821 Bankner Khair Aligarh Vayam Tech. 15 Ajay Pal Singh 9012148987 Kandli Iglas Aligarh Vayam Tech. 16 Puneet Agrawal 8410104219 Chota Jawan Jawan Aligarh Vayam Tech. 17 Upendra Singh 9568154697 Nagla Lochan Bijoli Aligarh Vayam Tech. 18 VIKAS 9719632620 CHAK VEERUMPUR JEWAR G.B.Nagar Vayam Tech. 19 MUSARRAT ALI 9015072930 JARCHA DADRI G.B.Nagar Vayam Tech. 20 SATYA BHAN SINGH 9818498799 KHATANA DADRI G.B.Nagar Vayam Tech. 21 SATYVIR SINGH 8979997811 NAGLA NAINSUKH DADRI G.B.Nagar Vayam Tech. 22 VIKRAM SINGH 9015758386 AKILPUR JAGER DADRI G.B.Nagar Vayam Tech. 23 Pushpendra Kumar 9412845804 Mohmadpur Jadon Dankaur G.B.Nagar Vayam Tech. 24 Sandeep Tyagi 9810206799 Chhaprola Bisrakh G.B.Nagar Vayam Tech. -

ASHA Data Base Kanpur Dehat Name of ID No.Of Population S.No
ASHA Data Base Kanpur Dehat Name Of ID No.of Population S.No. Name Of Block Name Of CHC/BPHC Name Of Sub-Centre Name Of ASHA Husband's Name Name Of Village District ASHA Covered 1 2 3 4 5 6 7 8 9 10 1 Kanpur Dehat AMRAUDHA PHC AMRAUDHA RURGANV 4202001 ALKA OMPRAKASH DHIKCHI 1150 2 Kanpur Dehat AMRAUDHA PHC AMRAUDHA SHEKHPUR 4202002 ANITA KARTAR SINGH MAHKAPUR 1035 3 Kanpur Dehat AMRAUDHA PHC AMRAUDHA MUSHANAGAR 4202003 ANITA SHIUV KUMAR GAUSHGANJ 900 GAUSHGANJ 4 Kanpur Dehat AMRAUDHA PHC AMRAUDHA MUSHANAGAR 4202004 ANURADHA PRADEEP KUMAR GAUSHGANJ 1000 GAUSHGANJ 5 Kanpur Dehat AMRAUDHA PHC AMRAUDHA MUSHANAGAR BANGAR 4202005 ARADHANA RAJBAHADUR KRIPALPUR 1000 6 Kanpur Dehat AMRAUDHA PHC AMRAUDHA CHAKCHALPUR 4202006 ARCHANA RAJESH DURGDASPUR 862 7 Kanpur Dehat AMRAUDHA PHC AMRAUDHA BARAULI 4202007 ASHA DEVI KAMTA PRASHAD JALLAPUR 1797 8 Kanpur Dehat AMRAUDHA PHC AMRAUDHA CHAURA 4202008 ASHA DEVI NARENDRA RAO JALPURA 1000 9 Kanpur Dehat AMRAUDHA PHC AMRAUDHA MACHA 4202009 ASHA DEVI RAJ KUMAR MAUKHAS 1400 10 Kanpur Dehat AMRAUDHA PHC AMRAUDHA SHAHJAHANPUR 4202010 ASHA DEVI SANTOSH KUMAR BADI BAHERI 600 11 Kanpur Dehat AMRAUDHA PHC AMRAUDHA MUSHANAGAR 4202011 ASHA VIJAY NARAYAN GAUSHGANJ 750 GAUSHGANJ 12 Kanpur Dehat AMRAUDHA PHC AMRAUDHA CHAURA 4202012 ASHA DEVI YASHWANT KUMAR PIPARI 1000 13 Kanpur Dehat AMRAUDHA PHC AMRAUDHA BHOGANIPUR 4202013 AVADHESH KUMARI VINOD KUMAR BHOGANIPUR 1300 14 Kanpur Dehat AMRAUDHA PHC AMRAUDHA PAREHARAPUR 4202014 BEENA ANIL MEERPUR 3662 15 Kanpur Dehat AMRAUDHA PHC AMRAUDHA DADHWAPUR 4202015 BHAGYAVATI RAJKISHOR -

Agra North Agra Kiraoli Etmadpur Agra South
NAME OF THE DISTRICT TALUKS COVERED NAME OF THE CO-ORDINATOR CONTACT NUMBER EMAIL-ID AGRA NORTH AGRA KIRAOLI ETMADPUR AGRA SOUTH KHERAGARH FATEHABAD BAH ALIGARH NORTH KHAIR GABHANA IGLAS ALIGARH SOUTH KOIL ATRAULI ALLAHABAD NORTH ALLAHABAD SORAON HANDIA PHULPUR ALLAHABAD SOUTH KORAON MEJA KARCHHANA BARA AMBEDKAR NAGAR NORTH AKBARPUR BHITI TANDA AMBEDKAR NAGAR SOUTH JALALPUR ALLAPUR AMETHI AMETHI AMROHA AMROHA AURAIYA NORTH AURAIYA AURAIYA SOUTH BIDHUNA AZAMGARH NORTH BURHANPUR PHULPUR LALGANJ NIZAMABAD AZAMGARH SOUTH MEHNAGAR SAGRI AZAMGARH BAGPHAT NORTH BAGHPAT BARAUT BAGPHAT SOUTH KHEKADA BAHRAICH NORTH MAHASI NANPARA BAHRAICH SOUTH BAHRAICH KAISERGAJ BALLIA NORTH BELTHARA ROAD SIKANDERPUR RASRA BALLIA SOUTH BANSDIH BALLIA BAIRIA BALRAMPUR NORTH BALRAMPUR UTRAULA BALRAMPUR SOUTH TULSIPUR BANDA NORTH BANDA BABERU BANDA SOUTH NARAINI ATARRA BARABANKI NORTH FATEHPUR RAMNAGAR NAWABGANJ BARABANKI SOUTH RAMSANEHIGHAT SIRAULI GAUSPUR HAIDARGARH BAREILLY NORTH AONLA MEERGANJ BAREILLY BAREILLY SOUTH FARIDPUR NAWABGANJ BAHERI BASTI NORTH BHANPUR HARRAIYA BASTI SOUTH RUDHAULI BASTI BIJNOR NORTH BIJNOR NAGINA NAJIBABAD BIJNOR SOUTH DHAMPUR CHANDPUR BUDAUN NORTH BILSI GUNNAUR SAHASWAN BUDAUN SOUTH BUDAUN DATAGANJ BISAULI BULANDSHAHR NORTH SIKANDRABAD BULANDSHAHAR KHURJA SHIKARPUR BULANDSHAHR SOUTH SIANA DEBAI ANUPSHAHR CHANDAULI NORTH CHAKIA SAKALDIHA CHANDAULI SOUTH CHANDAULI CHITRAKOOT NORTH KARWI CHITRAKOOT SOUTH MAU DEORIA NORTH BARHAJ RUDRAPUR DEORIA DEORIA SOUTH SALEMPUR BHATPAR RANI ETAH NORTH JALESAR ETAH ETAH SOUTH ALIGANJ ETAWAH NORTH -

Weekly Disposal Report Fsl, Lucknow
WEEKLY DISPOSAL REPORT FSL, LUCKNOW Week Starting From - 08.03.2016 To 11.03.2016 SEROLOGY/DNA DIVISION SI. District Name Police Station Crime No. Date Of Date Of Remark No. Name Receipt Disposal 1. GHAZIABAD LONI BORDER 49/16 08.03.16 08.03.16 R.I.O. 2. GHAZIABAD LONI BORDER 94/16 08.03.16 08.03.16 R.I.O. 3. PILIBHIT JAHANABAD 78/16 08.03.16 08.03.16 R.I.O. 4. JALAUN AARA 05/16 09.03.16 09.03.16 R.I.O. 5. MAU DOHARIGHAT 89/16 09.03.16 09.03.16 R.I.O. 6. ALLAHABAD CIVIL LINE 94/16 10.03.16 10.03.16 R.I.O. 7. JALAUN KOTWALI URAI 215/16 11.03.16 11.03.16 R.I.O. 8. SAHJHANPUR BANDA 231/16 11.03.16 11.03.16 R.I.O. 9. MAU SOUTH TOLA 451/15 11.03.16 11.03.16 R.I.O. 10. MAU SOUTH TOLA 453/15 11.03.16 11.03.16 R.I.O. 11. SULTANPUR CHANDA 37/16 11.03.16 11.03.16 R.I.O. 12. ALLAHABAD INDUSTRIAL AREA 230/15 09.03.16 09.03.16 R.I.O. 13. ALLAHABAD CIVIL LINE 74/16 10.03.16 10.03.16 R.I.O. 14. SITAPUR RAMPUR KALA 44/16 10.03.16 10.03.16 R.I.O. 15. KANPUR NAGAR KALYANPUR 1223/15 11.03.16 11.03.16 R.I.O. WEEKLY DISPOSAL REPORT FSL, LUCKNOW Week Starting From - 08.03.2016 To 11.03.2016 CHEMISTRY DIVISION SI. -
E-Auction on 24-07-2019 ROSARB, Kanpur Branch : 2Nd Floor, 118/330, Gumti No
e-Auction on 24-07-2019 ROSARB, Kanpur Branch : 2nd Floor, 118/330, Gumti No. 5, Kaushalpuri, Kanpur-208 012 Ph. : 0512-2215173 • e-mail : [email protected] Sale Notice For Immovable Properties [ See Proviso to rule 8 (6) ] E-Auction Sale Notice for Sale of Immovable assets under the securitisation and Reconstruction of Financial assets and Enforcement of Security Interest Act, 2002 Read with Proviso to Rule 8 (6) of the Security Interest (Enforcement) Rules, 2002. Notice is hereby given to the public in general and in particular to the Borrower(s) and Guarantor(s) that the below described immovable property mortgaged/charged to the secured creditor, the constructive possession of which has been taken by the authorised officer of Bank of Baroda as Secured Creditor, will be sold on ‘‘As is Where is,’’ ‘‘As is What is,’’ and ‘‘Whatever there is’’ basis on Dated 24-07-2019 for recovery of bank's dues from Borrower(s) and Guarantor(s) to the secured creditor. The details are described as below. Last Date of EMD/Document is 23-07-2019 upto 4.00 pm Reserve Price Sl. Name & Address of the borrower & Guarantor / Description of Property A/c No. for Deposit Earnest Money e-Auction Deposit / Bid Date & No. & Date of Demand & Possession / Amount of Debt of EMD / BID Amount Increment Amount Time Branch : ROSARB, Kanpur NO LIEN A/c ROSARB KANPUR IFSC Code : BARB0GUMTIX Mob. : 8601875603, 8601875614 No. : 12820200001407 please read "0" as "zero" 1. Borrower : M/s Jai Durge Brick Fields, Address : Village Bindhela Salepur, Bindki, Fatehpur. -

07 12 2015 3.Pdf
VTP Center Registration Center State Center District Name of Center Center Address Center City CenterEmail CenterTelephone Center Mobile Courses PinCode Number No 209150001 UTTAR PRADESH Agra GITI Agra Balkeshwar Road Agra 282004 Agra 282004 [email protected] 0562-2541385 9628374364 209150003 UTTAR PRADESH Agra GITI Etmadpur, Agra Village-Agwar Tehsil-Etmadpur Agra Agra 282001 [email protected] 0562-2540050 8532865560 209150002 UTTAR PRADESH Agra GITI WB Women Agra Govt. ITI Campus Balkeshwar Agra Agra 282004 [email protected] 0562-2542255 9412596138 GOVERNMENT LEATHER INSTITUTE, AGRA GOVERNMENT LEATHER INSTITUTE, NUNIHAI, LEA101, 209150004 UTTAR PRADESH Agra RAMBAGH, AGRA AGRA 282006 [email protected] 0562-2281104 9411939981 Govt. Industrial Training Institute Bah Agra , Agra Situated At Govt. Industrial Training Institute Campus 209150005 UTTAR PRADESH Agra Balkeshwar Agra Agra 282004 [email protected] 0562-2542255 9997753566 209120001 UTTAR PRADESH Aligarh GITI Aligarh Govt. Industrial Training Institute Aligarh Aligarh 202001 [email protected] 0571-2405203 9628474362 209120002 UTTAR PRADESH Aligarh GITI Atrauli, Aligarh Atrauli Aligarh Atrauli 202280 [email protected] 09837-469908 8532865560 GOVT. INDUSTRIAL TRAINING INSTITUTE, KOIL GOVT. I.T.I.- KOIL, NEAR NAWAB SINGH CHAUHAN GRAMODAYA INTER COLLEGE, KASIMPUR POWER 209120003 UTTAR PRADESH Aligarh HOUSE, ALIGARH ALIGARH 202127 [email protected] 0571-2904455 9760554665 Rathi Hospital , Aligarh HIG-6 Vikas Nagar, Agra Road, Aligarh MED101,MED102,MED1 209120004 UTTAR PRADESH Aligarh Aligarh -

District Census Hand Book, Kanpur, Part-XIII A, Series-22, Uttar Pradesh
CENSUS 1981 ~lrr XIII - 3f ff)~SERIES-22 tJl~ ~~~ i{rr~ ...... a-m~ f;:r(l~;ft UTTAR PRADESH Part XIII- A VillAGE & TOWN DIRECTORY ~T ~ifflOf,;{T DISTRICT ~fcl ~ ft:cl otT I<ANPUR- DISTRICT CEl~SUS HANDBOOK ~.niii 1).t o, +trorJ11 ~m~f.rr. U<JT fif~, iifif~r tIT0fT<'fii, ~n,ml ---- 79- lO' ""' 'S 80' IS 30' I~ 21 27 DISTRICT KANPUR 5 0 10 15 >0 '~Km ~~~ , v ~ ., ~ /' --45 ~.- ... "\ 1- ) pTO R.4SULABAD ~ ~ ~ \,-e)~.f 1- ~ I. ~ 3() ,,; ~ "1 '0" "). 0 .. o v 15 t. BOUNDARIES - DISTRICT. TAHSIL. VIr;ASI(HANO J.lIGHWAYS:- NATIONAL) STATE ••••.••••••• ~N~H~2 _____ .~S~H~/7____ _ IMPORTANT METALLED ROAD •• RAILWAY LINE WITH SH.TIONS - BROAD GAUGEtrt:J.I£TRE GAUGE •• Ri".....__, 1IIIIiiililtili RIVER AND STR£AN ••• .............................. ~~ HCADQuiRTERS - DISTRICT ~ TAHSIL .VIKASKHAfiD ••••••••• @.~.O Siz~ C1t?ssts 01 Urban Centres lJRPAlV CENTRE, •• ......... " ••••••••.•••••••••.•.. o VILLAGE HAVING :5000 & A80VE P(JPULAT!ON WITH NAME , , CLASS IA POST AND TELEGRAPH OFFICE ••• •• DEGREE COLLEGE ,., •• _ ••• o ..' IV TECHNICAL INSTITUTION GJ " V 8UNGALOWS - !NSPECTION ••• 18 RESTHOI.,;SE ••• '" •••••••• RH East Of Grunw;c/"> 4S' .0· Is' BA SED UP,ON SURVEY OF INDIA ~AP WITH THE PERMISSION OF THE SURVEYOR GENERAL OF INDIA @ GOVERNMENT OF IFIO''A COPYRIGHT Ig&J P.$. U.P. (ReJ 1 JANGANANA!127- 23-7-95 -1.550. i. SI~ ICli11 2. ~ v 3. ~ifiT~ 4. f~t~~ Ix S. ~ GwIUIi11 ii'RI~f~'fir ~ Ifft;:rlr xiii 6. fCjQ?t~UII~fj'fi ~1 (a:(~;;ft ij) 1. ~ I-m+T ~fu'fiT, 19 -386 ~: l.'f~h 23-82 (i)~~ (ii) !lTtrT ctI" ~ililr ~1 24 (iii) m+T f"1~flll<fir 34 2. -

Kanpur Dehat Page:- 1 Cent-Code & Name Exam Sch-Status School Code & Name #School-Allot Sex Part Group 1001 Akbarpur I C Akbarpur Kanpur Dehat Bum
DATE:27-02-2021 BHS&IE, UP EXAM YEAR-2021 **** FINAL CENTRE ALLOTMENT REPORT **** DIST-CD & NAME :- 39 KANPUR DEHAT PAGE:- 1 CENT-CODE & NAME EXAM SCH-STATUS SCHOOL CODE & NAME #SCHOOL-ALLOT SEX PART GROUP 1001 AKBARPUR I C AKBARPUR KANPUR DEHAT BUM HIGH BUM 1001 AKBARPUR I C AKBARPUR KANPUR DEHAT 190 F HIGH BUF 1008 AKBARPUR GIRLS IC AKBARPUR KANPUR DEHAT 96 F HIGH CRM 1151 GRAMIN H S S BARA KANPUR DEHAT 73 M HIGH CRM 1158 SRIKRISHNA BHIKHAM SINGH SWATANTRATA SANGRAM SENANI I C M 47 M HIGH CRM 1185 BHARTIYA GYAN STHALI GAZNER RD JAINPUR KANPUR DEHAT 29 F HIGH CUM 1312 S V P S S S AKBARPUR KANPUR DEHAT 4 F 439 INTER BUM 1001 AKBARPUR I C AKBARPUR KANPUR DEHAT 78 F OTHER THAN SCICNCE INTER BUM 1001 AKBARPUR I C AKBARPUR KANPUR DEHAT 147 F SCIENCE INTER CRM 1142 J S S I C MATI KISHANPUR KANPUR DEHAT 156 M SCIENCE INTER CRM 1161 STNGSIC JAINPUR KANPUR DEHAT 44 F ALL GROUP INTER CRM 1303 CH GAYAPRASAD INTER COLLEGE KUIT KHERA AKBARPUR KANPUR DEHAT 6 M OTHER THAN SCICNCE INTER BUM 5001 AKBARPUR I C AKBARPUR KANPUR DEHAT 2 F ALL GROUP 433 CENTRE TOTAL >>>>>> 872 1002 R P S INTER COLLEGE RURA KANPUR DEHAT BUM HIGH BUM 1002 R P S INTER COLLEGE RURA KANPUR DEHAT 39 F HIGH BRM 1007 R S I C BHIKHANAPUR KANPUR DEHAT 65 M HIGH BRM 1066 GALUVAPUR I C GALUVAPUR KANPUR DEHAT 104 M HIGH CUM 1155 VEENA VADINEE HSS RURA KANPUR DEHAT 22 M HIGH CRM 1173 M L INTER COLLEGE DAGARAHA BANIPARA KANPUR DEHAT 124 M HIGH CRM 1192 PT RAMJI LAL INTER COLLEGE BANIPARA KANPUR DEHAT 35 M HIGH CRM 1225 PT H N S I C BANIPARA KANPUR DEHAT 89 M HIGH CRM 1290 UMASHANKAR MEERA -
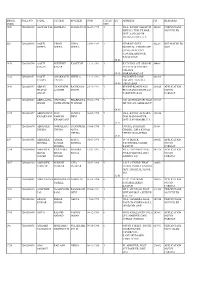
Image Code Roll No Name Father Mother Dob Categ
IMAGE ROLL NO NAME FATHER MOTHER DOB CATEG EQ ADDRESS PIN REMARKS CODE ORY 1549 7260200430 AADESH PAL SHRIRAM SEEMA DEVI 06-07-1992 1 VILL. & POST SAHAPUR 242301 PERCENTAGE KHITAU, TEH. TILHAR, NOT FILLED DIST. SAHAJAPUR, THANA KATRA, U.P. 358 7260200095 AARTI VINOD USHA 21-09-1989 9 NEAR BY GOVT. 322201 BSC MATHS EQ GUPTA GUPTA GUPTA HOSPITAL, CHULIGATE NOT GANGAPUR, CITY, SAWAIMADHOPUR, RAJASTHAN 80.88 2470 7260200754 AARTI SUKHDEV KAMLESH 11-11-1993 1 KATGHAR GULAB BARI 244001 SAGAR SAGAR JATAV BASTI INDRA COLONY 65.18 MORADABAD,UP 1322 7260200351 AARTI AMARNATH SHEELA 13-11-1991 6 606 D REST CAMP 283204 YADAV YADAV COLONY, TUNDLA, 64.00 FIROZABAD 1436 7260200397 ABHAY GYANENDR RAVIKALA 25-11-1991 9 BEHIND ROADWAYS 209625 APPLICATION PRATAP A SINGH SINGH BUS STAND DIGGITAAL NOT IN SINGH FARRUKHABAD FORMAT 202 7260200053 ABHILASHA UPENDRA PRABHAWA 09-08-1990 9 G.D 250TRIVAINIPURAM 211019 SINGH NATH SINGH TI SINGH JHUNSI ALLAHABAD UP 60.90 1725 7260200497 ABHIMANYU SHIV SUNITA 03-07-1992 9 VILL. & POST ALWARA, 212214 KESARVANI NARESH DEVI TEH. MANJHANPUR, KESARVANI DIST. KAUSHAMBI, U.P. 57.25 275 7260200074 ABHISHEK OMRAKASH CHANDRAK 15-08-1988 9 CHURA, SUBHASH 33100 CHURA CHURA ANTA CHOWK, OJHA KI GALI CHURA CHURU RAJASTHAN 65.53 603 7260200152 ABHISHEK ASHOK MAYA 01-03-1990 9 18- 'O' BLOCK 208011 APPLICATION MISHRA KUMAR MISHRA YASWHODA NAGAR NOT IN MISHRA KANPUR FORMAT 1714 7260200490 ABHISHEK RAJENDRA SHODRA 12-01-1991 1 VILL. GAUSINGH PUR, 241126 APPLICATION SINGH SINGH SINGH PO KACHHONIA, DIST NOT IN TOMER TOMER HARDOI, U.P. -
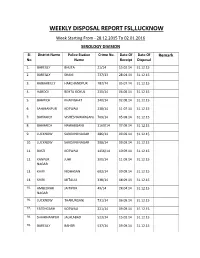
WEEKLY DISPOSAL REPORT FSL,LUCKNOW Week Starting from - 28.12.2015 to 02.01.2016 SEROLOGY DIVISION
WEEKLY DISPOSAL REPORT FSL,LUCKNOW Week Starting From - 28.12.2015 To 02.01.2016 SEROLOGY DIVISION SI. District Name Police Station Crime No. Date Of Date Of Remark No. Name Receipt Disposal 1. BAREILLY BHUTA 21/14 15.02.14 31.12.15 2. BAREILLY SHANI 737/13 28.04.14 31.12.15 3. RAIBAREILLY HARCHANDPUR 182/14 05.07.14 31.12.15 4. HARDOI BEHTA GOKUL 230/14 06.08.14 31.12.15 5. BAHRICH KHAINGHAT 340/14 02.08.14 31.12.15 6. SAHJHANPUR KOTWALI 238/14 11.07.14 31.12.15 7. BAHRAICH VISHESHAWARGANJ 769/14 05.08.14 31.12.15 8. BAHRAICH NAWABGANJ 1160/14 07.08.14 31.12.15 9. LUCKNOW SAROJINI NAGAR 286/14 09.09.14 31.12.15 10. LUCKNOW SAROJINI NAGAR 308/14 09.09.14 31.12.15 11. BASTI KOTWALI 1456/14 10.09.14 31.12.15 12. KANPUR JUHI 300/14 11.09.14 31.12.15 NAGAR 13. KHIRI NIDHASAN 602/14 09.09.14 31.12.15 14. KHIRI MITAULI 338/14 08.09.14 31.12.15 15. AMBEDKAR JAITIPUR 49/14 28.04.14 31.12.15 NAGAR 16. LUCKNOW THAKURGANJ 731/14 06.09.14 31.12.15 17. FATEHGARH KOTWALI 221/14 09.09.14 31.12.15 18. SHAHJHANPUR JALALABAD 523/14 15.02.14 31.12.15 19. BAREILLY BAHERI 537/14 09.09.14 31.12.15 20. LUCKNOW THAKURGANJ 657/14 06.09.14 31.12.15 21. SHRAWASTI KOTWALI BHINGA 1291/14 08.09.14 31.12.15 22.