Eco–Immunology of Native and Invasive Water Bugs: Insights from Phenoloxidase Activity
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Metacommunities and Biodiversity Patterns in Mediterranean Temporary Ponds: the Role of Pond Size, Network Connectivity and Dispersal Mode
METACOMMUNITIES AND BIODIVERSITY PATTERNS IN MEDITERRANEAN TEMPORARY PONDS: THE ROLE OF POND SIZE, NETWORK CONNECTIVITY AND DISPERSAL MODE Irene Tornero Pinilla Per citar o enllaçar aquest document: Para citar o enlazar este documento: Use this url to cite or link to this publication: http://www.tdx.cat/handle/10803/670096 http://creativecommons.org/licenses/by-nc/4.0/deed.ca Aquesta obra està subjecta a una llicència Creative Commons Reconeixement- NoComercial Esta obra está bajo una licencia Creative Commons Reconocimiento-NoComercial This work is licensed under a Creative Commons Attribution-NonCommercial licence DOCTORAL THESIS Metacommunities and biodiversity patterns in Mediterranean temporary ponds: the role of pond size, network connectivity and dispersal mode Irene Tornero Pinilla 2020 DOCTORAL THESIS Metacommunities and biodiversity patterns in Mediterranean temporary ponds: the role of pond size, network connectivity and dispersal mode IRENE TORNERO PINILLA 2020 DOCTORAL PROGRAMME IN WATER SCIENCE AND TECHNOLOGY SUPERVISED BY DR DANI BOIX MASAFRET DR STÉPHANIE GASCÓN GARCIA Thesis submitted in fulfilment of the requirements to obtain the Degree of Doctor at the University of Girona Dr Dani Boix Masafret and Dr Stéphanie Gascón Garcia, from the University of Girona, DECLARE: That the thesis entitled Metacommunities and biodiversity patterns in Mediterranean temporary ponds: the role of pond size, network connectivity and dispersal mode submitted by Irene Tornero Pinilla to obtain a doctoral degree has been completed under our supervision. In witness thereof, we hereby sign this document. Dr Dani Boix Masafret Dr Stéphanie Gascón Garcia Girona, 22nd November 2019 A mi familia Caminante, son tus huellas el camino y nada más; Caminante, no hay camino, se hace camino al andar. -

Position Specificity in the Genus Coreomyces (Laboulbeniomycetes, Ascomycota)
VOLUME 1 JUNE 2018 Fungal Systematics and Evolution PAGES 217–228 doi.org/10.3114/fuse.2018.01.09 Position specificity in the genus Coreomyces (Laboulbeniomycetes, Ascomycota) H. Sundberg1*, Å. Kruys2, J. Bergsten3, S. Ekman2 1Systematic Biology, Department of Organismal Biology, Evolutionary Biology Centre, Uppsala University, Uppsala, Sweden 2Museum of Evolution, Uppsala University, Uppsala, Sweden 3Department of Zoology, Swedish Museum of Natural History, Stockholm, Sweden *Corresponding author: [email protected] Key words: Abstract: To study position specificity in the insect-parasitic fungal genus Coreomyces (Laboulbeniaceae, Laboulbeniales), Corixidae we sampled corixid hosts (Corixidae, Heteroptera) in southern Scandinavia. We detected Coreomyces thalli in five different DNA positions on the hosts. Thalli from the various positions grouped in four distinct clusters in the resulting gene trees, distinctly Fungi so in the ITS and LSU of the nuclear ribosomal DNA, less so in the SSU of the nuclear ribosomal DNA and the mitochondrial host-specificity ribosomal DNA. Thalli from the left side of abdomen grouped in a single cluster, and so did thalli from the ventral right side. insect Thalli in the mid-ventral position turned out to be a mix of three clades, while thalli growing dorsally grouped with thalli from phylogeny the left and right abdominal clades. The mid-ventral and dorsal positions were found in male hosts only. The position on the left hemelytron was shared by members from two sister clades. Statistical analyses demonstrate a significant positive correlation between clade and position on the host, but also a weak correlation between host sex and clade membership. These results indicate that sex-of-host specificity may be a non-existent extreme in a continuum, where instead weak preferences for one host sex may turn out to be frequent. -
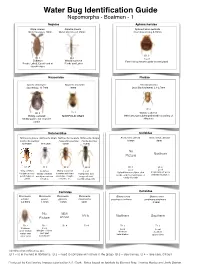
Water Bug ID Guide
Water Bug Identification Guide Nepomorpha - Boatmen - 1 Nepidae Aphelocheridae Nepa cinerea Ranatra linearis Aphelocheirus aestivalis Water Scorpion, 20mm Water Stick Insect, 35mm River Saucer bug, 8-10mm ID 1 ID 1 ID 1 Local Common Widely scattered Fast flowing streams under stones/gravel Ponds, Lakes, Canals and at Ponds and Lakes stream edges Naucoridae Pleidae Ilycoris cimicoides Naucoris maculata Plea minutissima Saucer bug, 13.5mm 10mm Least Backswimmer, 2.1-2.7mm ID 1 ID 1 ID 2 Widely scattered Widely scattered NORFOLK ONLY Often amongst submerged weed in a variety of Muddy ponds and stagnant stillwaters canals Notonectidae Corixidae Notonecta glauca Notonecta viridis Notonecta maculata Notonecta obliqua Arctocorisa gemari Arctocorisa carinata Common Backswimmer Peppered Backswimmer Pied Backswimmer 8.8mm 9mm 14-16mm 13-15mm 15mm 15mm No Northern Picture ID 2 ID 2 ID 2 ID 2 ID 3 ID 3 Local Local Very common Common Widely scattered Local Upland limestone lakes, dew In upland peat pools Ubiquitous in all Variety of waters In waters with hard Peat ponds, acid ponds, acid moorland lakes, or with little vegetation ponds, lakes or usually more base substrates, troughs, bog pools and sandy silt ponds canals rich sites concrete, etc. recently clay ponds Corixidae Corixidae Micronecta Micronecta Micronecta Micronecta Glaenocorisa Glaenocorisa scholtzi poweri griseola minutissima propinqua cavifrons propinqua propinqua 2-2.5mm 1.8mm 1.8mm 2mm 8.3mm No NEW RARE Northern Southern Picture ARIVAL ID 2 ID 2 ID 4 ID 4 ID 3 ID 3 Local Common Local Local Margins of rivers open shallow Northern Southern and quiet waters over upland lakes upland lakes silt or sand backwaters Identification difficulties are: ID 1 = id in the field in Northants. -

Heteroptera: Nepomorpha, Gerromorpha) in Portugal, with the Review of Biology of the Nearctic Corixid Trichocorixa Verticalis (Fieber, 1851)
Boletín Sociedad Entomológica Aragonesa, n1 38 (2006) : 359−361. NOTAS BREVES A contribution to the faunistics of aquatic and semiaquatic bugs (Heteroptera: Nepomorpha, Gerromorpha) in Portugal, with the review of biology of the Nearctic corixid Trichocorixa verticalis (Fieber, 1851) Petr Kment Department of Entomology, National Museum, Kunratice 1, CZ-148 00 Praha 4; Czech Republic & Department of Zoology, Fac- ulty of Science, Charles University, Viničná 7, CZ-128 44 Praha 2, Czech Republic − [email protected]. Abstract: Several small samples of aquatic and semiaquatic bugs (Heteroptera: Nepomorpha, Gerromorpha) collected mainly in southern Portugal in 2004 were examined. Altogether 20 species were identified, including two species of halobiont Corixidae – Sigara stagnalis stagnalis (Leach, 1817) and alien Trichocorixa verticalis verticalis (Fieber, 1851) – just recently discovered in Portugal. Distribution, ecology and the life cycle of T. v. verticalis are reviewed. Key words: Corixidae, Naucoridae, Nepidae, Notonectidae, Pleidae, Gerridae, Hebridae, Mesoveliidae, alien species, Portugal Introduction Despite several contributions published in the last decades, the true tum, fresh water, 8.xi.2004, M. Mantič lgt. 13) 1 km E of Junqueira, bug fauna of Portugal is still insufficiently known. The aquatic and Vila Real de Sto. António env. (37°15'15"N, 07°27'37"W), 2.- semiaquatic Heteroptera were listed in papers by de Seabra (1941), 4.iv.2004, J. Skuhrovec lgt. Serrao-Nogueira & Azevedo y Silva (1970), Nieser & Montes WESTERN PORTUGAL, PROVINCIA DO SANTARÉM, SANTARÉM REG.: 14) (1984), Baena & Vázquez (1986), and Aukema & Rieger (1995). Parque Natural de la Sierra d´Aire en Candeiros, Monsante (20 km Additional information, including exact records and descriptions of N of Santarém), at light, 19.-21.vi.2005, Z. -

Aquatic and Semiaquatic True Bugs (Hemiptera
Analele Universităţii din Oradea - Fascicula Biologie Tom. XVIII, Issue: 1, 2011, pp. 29-33 AQUATIC AND SEMIAQUATIC TRUE BUGS (HETEROPTERA: NEPOMORPHA) OF CEFA NATURE PARK (NORTH-WESTERN ROMANIA) Gavril Marius BERCHI*, Milca PETROVICI*, Daniela Minodora ILIE** * West University of Timişoara, Faculty of Chemistry, Biology and Geography, Department of Biology, Timişoara, Romania **Lucian Blaga University of Sibiu, Faculty of Sciences, Department of Ecology and Environmental Protection, Sibiu, România Corresponding author: Marius Berchi, West University of Timişoara, Faculty of Chemistry, Biology and Geography, Department of Biology, 16A Pestalozzi, 300115, Timişoara, România, phone: 0040767535056, fax: 0040256592620, e-mail: [email protected] Abstract: This study aims to complete the aquatic and semiaquatic Heteroptera (Hemiptera: Heteroptera: Nepomorpha) list of species for Cefa Nature Park. Specimens were collected by monthly monitoring different types of habitats in the protected area. The study reveals an addition of 17 species, along with the 8 species previously described for this area. Was noted the presence of Aphelocheirus aestivalis Fabricius, 1794 and Cymatia rogenhoferi Fieber, 1864 species that have a sporadic presence in the Romanian fauna, being reported few records for these species. These data complete the information on this group collected on the other side of the Romanian-Hungarian border, in Körös-Maros National Park, where a number of 28 species were identified. Because the goal is to create a protected area on both sides of the border, it is imperative to know this group in both protected areas, in order to create a common monitoring plan and common management measures. Keywords: aquatic bugs, Cefa Nature Park, Crişana, faunistics, Heteroptera, Nepomorpha, true bugs. -

423 – Aquatic Hemiptera of Northeastern
AQUATIC HEMIPTERA OF NORTHEASTERN ALGERIA: DISTRIBUTION, PHENOLOGY AND CONSERVATION Fouzi ANNANI 1,2, Ahmed H. AL F ARHAN 3 & Boudjéma SA M RAOUI 1,3* RÉSUMÉ.— Hémiptères aquatiques du nord-est de l’Algérie : distribution, phénologie et conserva- tion.— L’échantillonnage de 83 sites à travers le complexe de zones humides du nord-est Algérien, un point chaud de la biodiversité aquatique, a permis d’identifier 35 espèces d’hémiptères aquatiques. La répartition et la phénologie des espèces sont présentées et les histoires de vie de Notonecta glauca et Notonecta obli- qua déduites. Ces deux espèces estivent dans des milieux refuges à hautes altitudes avant de redescendre se reproduire en plaine à l’automne. Diverses manifestations de changements globaux (pompage de l’eau, construction de barrages, introduction d’espèces exotiques et fragmentation des milieux) influencent néga- tivement l’intégrité écologique des milieux de la région étudiée. SUMMARY.— A survey, involving the sampling of 83 sites, investigated the aquatic hemiptera of north- eastern Algeria, a well known hotspot of aquatic biodiversity. The study recorded 35 species with data on distribution and phenology presented and discussed. Aspects of the life history of some species (Notonecta glauca and Notonecta obliqua) were inferred from their distribution and phenology and they were found to aestivate at high altitude refuges. Insect conservation in North Africa is still embryonic, relying mainly on protected areas to provide surrogate conservation to a rich and diverse group. This is inadequate in view of the current distribution of aquatic insects, often located in unprotected habitats (intermittent streams, temporary pools, dunary ponds) and the fact that diverse manifestations of global changes (loss of habitats due to water extraction and dam construction, invasive species, habitat fragmentation) are fast eroding the biodiversity of protected areas. -

Heteroptera) Fauna of Azerbaijan Province, Iran
Turk J Zool 33 (2009) 421-431 © TÜBİTAK Research Article doi:10.3906/zoo-0804-3 Notes on the true bug (Heteroptera) fauna of Azerbaijan province, Iran Mohammad Ali GHARAAT1,*, Mohammad HASSANZADEH1, Mohammad Hasan SAFARALIZADEH1, Majid FALLAHZADEH2 1Department of Entomology, Agricultural Faculty, Urmia University, Urmia - IRAN 2Department of Entomology, Islamic Azad University, Jahrom Branch, Jahrom - IRAN Received: 07.04.2008 Abstract: The Heteroptera fauna in east Azerbaijan and west Azerbaijan provinces in northwestern Iran was surveyed during 2005-2006. In all, 73 species from 18 families were collected and identified, of which 1 species, Mozena lunata (Burmeister, 1835) (Coreidae), is a new record for the Palearctic ecozone and 6 species are newly recorded from Iran. Key words: Fauna, Heteroptera, Iran, Palearctic ecozone Introduction present study were to provide detailed information on The Heteroptera are very important in agriculture the distribution of Heteroptera in east Azerbaijan and (Linnavuori and Hosseini, 2000). In this suborder west Azerbaijan provinces, and to contribute to the there are aquatic, semi-aquatic, and terrestrial species, knowledge of the Iranian Heteroptera fauna. some of which are important agricultural and silvicultural pests (Kerzhner and Yachevski, 1964). On Materials and methods the other hand, predacious bugs reduce the number The study was conducted during 2005-2006, and of agricultural pests and may be used for biological nymph and adult specimens in the regions were control (Linnavuori and Hosseini, 2000); therefore, collected from different locations using different identification of Heteroptera is important (Linnavuori methods (Figure, and Tables 1 and 2). Most of the and Hosseini, 2000). specimens were collected by sweep net, light trap, or The Iranian Heteroptera fauna is rather well rectangular frame tray net. -

High-Altitude Migration of Heteroptera in Britain
Eur. J. Entomol. 110(3): 483–492, 2013 http://www.eje.cz/pdfs/110/3/483 ISSN 1210-5759 (print), 1802-8829 (online) High-altitude migration of Heteroptera in Britain 1, 2 3 2, 4 DON R. REYNOLDS , BERNARD S. NAU and JASON W. CHAPMAN 1 Natural Resources Institute, University of Greenwich, Chatham, Kent ME4 4TB, UK; e-mail: [email protected] 2 Rothamsted Research, Harpenden, Hertfordshire AL5 2JQ, UK 315 Park Hill, Toddington, Bedfordshire LU5 6AW, UK 4 Environment and Sustainability Institute, University of Exeter, Penryn, Cornwall TR10 9EZ, UK Key words. Heteropteran bugs, aerial sampling, windborne migration, atmospheric transport, life-history strategies, seasonal cycles Abstract. Heteroptera caught during day and night sampling at a height of 200 m above ground at Cardington, Bedfordshire, UK, during eight summers (1999, 2000, and 2002–2007) were compared to high-altitude catches made over the UK and North Sea from the 1930s to the 1950s. The height of these captures indicates that individuals were engaged in windborne migration over distances of at least several kilometres and probably tens of kilometres. This conclusion is generally supported by what is known of the spe- cies’ ecologies, which reflect the view that the level of dispersiveness is associated with the exploitation of temporary habitats or resources. The seasonal timing of the heteropteran migrations is interpreted in terms of the breeding/overwintering cycles of the spe- cies concerned. INTRODUCTION of ~200–300 km in eastern Australia (McDonald & Far- Migratory propensity is highly variable in the Hetero- row, 1988); and the huge numbers of Cyrtorhinus ptera: wing polymorphisms or polyphenisms are common lividipennis (Miridae) and Microvelia spp. -

The Diversity of Feeding Habits Recorded for Water Boatmen
EUROPEAN JOURNAL OF ENTOMOLOGYENTOMOLOGY ISSN (online): 1802-8829 Eur. J. Entomol. 114: 147–159, 2017 http://www.eje.cz doi: 10.14411/eje.2017.020 REVIEW The diversity of feeding habits recorded for water boatmen (Heteroptera: Corixoidea) world-wide with implications for evaluating information on the diet of aquatic insects CHRISTIAN W. HÄDICKE 1, *, DÁVID RÉDEI 2, 3 and PETR KMENT 4 1 University of Rostock, Institute of Biosciences, Chair of Zoology, Universitätsplatz 2, 18055 Rostock, Germany 2 Institute of Entomology, College of Life Sciences, Nankai University, Weijin Rd. 94, 300071 Tianjin, China; e-mail: [email protected] 3 Hungarian Natural History Museum, Department of Zoology, 1088 Budapest, Baross u. 13, Hungary 4 National Museum, Department of Entomology, Cirkusová 1740, CZ-193 00 Praha 9 – Horní Počernice, Czech Republic; e-mail: [email protected] Key words. Heteroptera, Corixoidea, aquatic invertebrates, food webs, diet, predation, feeding habits Abstract. Food webs are of crucial importance for understanding any ecosystem. The accuracy of food web and ecosystem models rests on the reliability of the information on the feeding habits of the species involved. Water boatmen (Corixoidea) is the most diverse superfamily of water bugs (Heteroptera: Nepomorpha), frequently the most abundant group of insects in a variety of freshwater habitats worldwide. In spite of their high biomass, the importance of water boatmen in aquatic ecosystems is fre- quently underestimated. The diet and feeding habits of Corixoidea are unclear as published data are frequently contradictory. We summarise information on the feeding habits of this taxon, which exemplify the diffi culties in evaluating published data on feeding habits in an invertebrate taxon. -

Hemiptera: Heteroptera) from India
ZOOLOGICAL SURVEY OF OCCASIONAL PAPER No. 273 RECORDS OF THE ZOOLOGICAL SURVEY OF INDIA A Synoptic List of Nepomorpba ( Hemiptera : Heteroptera ) from India G. THIRUMALAI Southern Regional Station, Zoological Survey of India J30, Santholne High Road, Chennai-28. Edited by the Director, Zoological Survey of India, Kolkata Zoological Survey of India Kolkata CITATION G. Thirumalai, 2007. A Synoptic List of Nepomorpha (Hemiptera: Heteroptera) from India. Rec. zool. Surv. India. Occ. Paper No., 273 : 1-84. (Published by the Director, zooI. Surv. India) Published : October, 2007 ISBN 978-81-8171-]73-1 © Govt. of India, 2007 ALL RIGHTS RESERVED • No part of this publication may be reporduced, stored in a retrieval system or transmitted, in any form or by any means, electronic, mechanical, photocopying, recording or otherwise withou the prior permission of the publisher. • This book is sold subject to the condition that it shall not, by way of trade, be lent, re-sold hired out or otherwise disposed of without the publisher's consent, in any form of binding or cover other than that in which it is published. • The correct price of this publication is the price printed on this page. Any revised price indicated by a rubber stamp or by a sticker or by any other means is incorrect and should be unacceptable. PRICE India Rs. 300 Foreign: $ 25 £ 20 Published at the Publication Division by the Director, Zoological Survey of India, 234/4, A.J.C. Bose Road, 2nd MSO Building, Nizam Palace (13th Floor), Kolkata-700 020 after laser type setting at Ghosh Enterprises, Kolkata-700 006 and printed at Krishna Printing Works, Kolkata 700 006. -

Autor Benslimane Et
Anthropogenic stressors are driving a steep decline of hemipteran diversity in dune ponds in north-eastern Algeria Nouara Benslimane, Khémissa Chakri, Dalal Haiahem, Anis Guelmami, Farrah Samraoui, Boudjéma Samraoui To cite this version: Nouara Benslimane, Khémissa Chakri, Dalal Haiahem, Anis Guelmami, Farrah Samraoui, et al.. An- thropogenic stressors are driving a steep decline of hemipteran diversity in dune ponds in north-eastern Algeria. Journal of Insect Conservation, Springer Verlag, 2019, 23 (3), pp.475-488. 10.1007/s10841- 019-00133-1. hal-02403092 HAL Id: hal-02403092 https://hal.archives-ouvertes.fr/hal-02403092 Submitted on 10 Dec 2019 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Anthropogenic stressors are driving a steep decline of hemipteran diversity in dune ponds in north-eastern Algeria Nouara Benslimane1,2 · Khémissa Chakri1,2 · Dalal Haiahem1 · Anis Guelmami3 · Farrah Samraoui1,4 ·Boudjéma Samraoui1,2 Boudjéma Samraoui : [email protected] 1 Laboratoire de Conservation des Zones Humides, Université 8 Mai 1945 Guelma, Guelma, Algeria 2 Department of Biology, University of Annaba, Annaba, Algeria 3 Tour du Valat, Research Institute for the Conservation of Mediterranean Wetlands, Tour du Valat, le Sambuc, 13200 Arles, France 4 Department of Ecology, Université 8 Mai 1945 Guelma, Guelma, Algeria How to cite : Benslimane N., Chakri K., Haiahem D., Guelmami A., Samraoui F., Samraoui B. -

(Hemiptera: Heteroptera) from Northern Iran 1991-1996 © Biologiezentrum Linz/Austria; Download Unter
ZOBODAT - www.zobodat.at Zoologisch-Botanische Datenbank/Zoological-Botanical Database Digitale Literatur/Digital Literature Zeitschrift/Journal: Linzer biologische Beiträge Jahr/Year: 2013 Band/Volume: 0045_2 Autor(en)/Author(s): Ghahari Hassan Artikel/Article: A study on aquatic and semiaquatic bugs (Hemiptera: Heteroptera) from northern Iran 1991-1996 © Biologiezentrum Linz/Austria; download unter www.biologiezentrum.a Linzer biol. Beitr. 45/2 1991-1996 20.12.2013 A study on aquatic and semiaquatic bugs (Hemiptera: Heteroptera) from northern Iran H. GHAHARI Abstract: Totally 19 species of aquatic and semiaquatic Heteroptera from the families Corixidae, Gerridae, Hydrometridae, Notonectidae, Saldidae were collected and identified from some regions of northern Iran, southern areas of Caspian Sea. Key words: Hemiptera, Heteroptera, Aquatic and semiaquatic bugs, Fauna, Iran. Introduction Insects represent the most diversified biological group within the arthropods, having occupied heterogeneous functional and environmental habitats. They are the most numerous animal and essential elements in most trophic and energetic chains (SANDWAYS 1994; FERNÁNDEZ & LÓPEZ RUF 2006). Heteroptera is a worldwide distributed group of insects inhabiting both terrestrial and aquatic habitats and has an important ecological role (SCHUH & SLATER 1995; NARANJO et al. 2010). Heteropteran species are a significant component of the aquatic fauna and play an important part in littoral food webs (NIESER 1975; SKERN et al. 2010). These insects are able to colonize all aquatic habitats, from small temporary ponds to lakes and rivers, from swamps to brackish waters, even if only temporarily during their migratory flights (NIESER 1978; BERCHI et al. 2011). Most species of the aquatic and semiaquatic Heteroptera belong to the infraorders Leptopodomorpha, Gerromorpha and Nepomorpha, with the last two encompassing approximately 92 % of the aquatic bugs diversity (ANDERSEN 1982; SCHUH & SLATER 1995; POLHEMUS & POLHEMUS 2008).