Art and Craft Safety Guide
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Treatise on Combined Metalworking Techniques: Forged Elements and Chased Raised Shapes Bonnie Gallagher
Rochester Institute of Technology RIT Scholar Works Theses Thesis/Dissertation Collections 1972 Treatise on combined metalworking techniques: forged elements and chased raised shapes Bonnie Gallagher Follow this and additional works at: http://scholarworks.rit.edu/theses Recommended Citation Gallagher, Bonnie, "Treatise on combined metalworking techniques: forged elements and chased raised shapes" (1972). Thesis. Rochester Institute of Technology. Accessed from This Thesis is brought to you for free and open access by the Thesis/Dissertation Collections at RIT Scholar Works. It has been accepted for inclusion in Theses by an authorized administrator of RIT Scholar Works. For more information, please contact [email protected]. TREATISE ON COMBINED METALWORKING TECHNIQUES i FORGED ELEMENTS AND CHASED RAISED SHAPES TREATISE ON. COMBINED METALWORKING TECHNIQUES t FORGED ELEMENTS AND CHASED RAISED SHAPES BONNIE JEANNE GALLAGHER CANDIDATE FOR THE MASTER OF FINE ARTS IN THE COLLEGE OF FINE AND APPLIED ARTS OF THE ROCHESTER INSTITUTE OF TECHNOLOGY AUGUST ( 1972 ADVISOR: HANS CHRISTENSEN t " ^ <bV DEDICATION FORM MUST GIVE FORTH THE SPIRIT FORM IS THE MANNER IN WHICH THE SPIRIT IS EXPRESSED ELIEL SAARINAN IN MEMORY OF MY FATHER, WHO LONGED FOR HIS CHILDREN TO HAVE THE OPPORTUNITY TO HAVE THE EDUCATION HE NEVER HAD THE FORTUNE TO OBTAIN. vi PREFACE Although the processes of raising, forging, and chasing of metal have been covered in most technical books, to date there is no major source which deals with the functional and aesthetic requirements -

The Dialogue of Craft and Architecture
University of Massachusetts Amherst ScholarWorks@UMass Amherst Masters Theses Dissertations and Theses July 2015 The Dialogue of Craft and Architecture Thomas J. Forker University of Massachusetts Amherst Follow this and additional works at: https://scholarworks.umass.edu/masters_theses_2 Part of the Architectural Technology Commons, and the Cultural Resource Management and Policy Analysis Commons Recommended Citation Forker, Thomas J., "The Dialogue of Craft and Architecture" (2015). Masters Theses. 197. https://doi.org/10.7275/7044176 https://scholarworks.umass.edu/masters_theses_2/197 This Open Access Thesis is brought to you for free and open access by the Dissertations and Theses at ScholarWorks@UMass Amherst. It has been accepted for inclusion in Masters Theses by an authorized administrator of ScholarWorks@UMass Amherst. For more information, please contact [email protected]. THE DIALOGUE OF CRAFT AND ARCHITECTURE A Thesis Presented by THOMAS J. FORKER Submitted to the Graduate School of the University of Massachusetts Amherst in partial fulfillment of the requirements for the degree of MASTER OF ARCHITECTURE MAY 2015 DEPARTMENT OF ARCHITECTURE THE DIALOGUE OF CRAFT AND ARCHITECTURE A Thesis Presented by THOMAS J. FORKER Approved as to style and content by: ___________________________ Kathleen Lugosch, Chair ___________________________ Ray Mann, Associate Professor ____________________ Professor Kathleen Lugosch Graduate Program Director Department of Architecture ____________________ Professor Stephen Schreiber Chair Department of Architecture DEDICATION This thesis is dedicated to my parents, for their love and support. ACKNOWLEDGMENTS I would like to thank my professors Kathleen Lugosch and Ray Mann. They have been forthright with their knowledge, understanding, and dedicated in their endeavor to work with the students in the department and in the pursuit of a masters of architecture degree with spirit and meaning. -

How to Apply Statuary and Patina Finishes
Working With Copper Soldering / Welding / Brazing How to Apply Statuary and Patina Finishes Copper and copper alloys are widely used in architectural applications to take ad- vantage of their inherent range of colors. While these metals may be used in their natural color, as fabricated, it is sometimes desirable to chemically color pure copper (C11000*), commercial bronze (C22000), architectural bronze (C38500) or other alloys referred to as “bronze” in architectural parlance. The most common colors to be produced are referred to as brown or statu- ary fi nishes for bronze and green or patina fi nishes for copper. This data sheet outlines procedures and formulations for producing both. While the chemical solu- tions described are those generally accepted in the metal fi nishing trade, many variations exist. The wide range of colors and shades which may be achieved are largely a matter of craftsmanship and experience. Chemical coloring techniques depend upon time, temperature, surface, preparation, mineral content of the water, humidity and other variables which infl uence the ultimate result. This data sheet presents the technology which underlies the craftsmanship and art involved in producing these colored fi nishes. Brown Statuary Finishes Statuary fi nishes are conversion coatings. In conversion coatings, the metal surface is either converted into a protective fi lm, usually an oxide or sulfi de of the metal involved, or a compound is precipitated which forms a surface fi lm. The use of chemical solutions is generally termed “oxidizing,” although the oldest method and the one which produces the widest range of brown to black stages on copper alloys actually produces not an oxide but a metal sulfi de fi nish by the use of alkaline sulfi de solutions. -
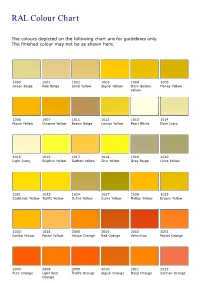
RAL Colour Chart
RAL Colour Chart The colours depicted on the following chart are for guidelines only. The finished colour may not be as shown here. 1000 1001 1002 1003 1004 1005 Green Beige Pale Beige Sand Yellow Signal Yellow Dark Golden Honey Yellow Yellow 1006 1007 1011 1012 1013 1014 Maize Yellow Chrome Yellow Brown Beige Lemon Yellow Pearl White Dark Ivory 1015 1016 1017 1018 1019 1020 Light Ivory Sulphur Yellow Saffron Yellow Zinc Yellow Grey Beige Olive Yellow 1021 1023 1024 1027 1028 1032 Cadmium Yellow Traffic Yellow Ochre Yellow Curry Yellow Mellon Yellow Broom Yellow 1033 1034 2000 2001 2002 2003 Dahlia Yellow Pastel Yellow Yellow Orange Red Orange Vermillion Pastel Orange 2004 2008 2009 2010 2011 2012 Pure Orange Light Red Traffic Orange Signal Orange Deep Orange Salmon Orange Orange 3000 3001 3002 3003 3004 3005 Flame Red RAL Signal Red Carmine Red Ruby Red Purple Red Wine Red 3007 3009 3011 3012 3013 3014 Black Red Oxide Red Brown Red Beige Red Tomato Red Antique Pink 3015 3016 3017 3018 3020 3022 Light Pink Coral Red Rose Strawberry Red Traffic Red Dark Salmon Red 3027 3031 4001 4002 4003 4004 Raspberry Red Orient Red Red Lilac Red Violet Heather Violet Claret Violet 4005 4006 4007 4008 4009 4010 Blue Lilac Traffic Purple Purple Violet Signal Violet Pastel Violet Telemagenta 5000 5001 5002 5003 5004 5005 Violet Blue Green Blue Ultramarine Blue dark Sapphire Black Blue Signal Blue Blue 5007 5008 5009 5010 5011 5012 Brilliant Blue Grey Blue Light Azure Blue Gentian Blue Steel Blue Light Blue 5013 5014 5015 5017 5018 5019 Dark Cobalt Blue -

Simple Chemical Experiments Simple Chemical Experiments
SIMPLE CHEMICAL EXPERIMENTS SIMPLE CHEMICAL EXPERIMENTS By ALFRED MORGAN Illustrated by THE AUTHOR APPLETON-CENTURY-CROFTS, INC. NEW YORK COPYRIGHT, 1941, BY D. APPLETON-CENTURY COMPANY, INC All rights reserved. This book, or parts thereof, must not be reproduced in any form without permission of the publisher. PRINTED IN THE UNITED STATES OF AMERICA CONTENTS CHAPTER PAGE I. YOUR LABORATORY i II. EXPERIMENTS WITH PRECIPITATES .... 26 III. EXPERIMENTS WITH SULFUR AND SOME OF ITS COMPOUNDS 54 IV. EXPERIMENTS WITH OXYGEN AND OXYGEN COM POUNDS 73 V. EXPERIMENTS WITH GASES AND SOME OF THEIR COMPOUNDS 103 VI. CHEMICAL TESTS 123 VII. SAFE "FIREWORKS" 144 VIII. EXPERIMENTS WITH A FEW ORGANIC COMPOUNDS 156 IX. CHEMICAL TRICKS AND MAGIC 170 X. MISCELLANEOUS EXPERIMENTS 186 XI. PRACTICAL USES FOR YOUR CHEMICAL KNOWL EDGE 214 XII. THE CHEMICALS YOU WILL NEED . .231 INDEX OF CHEMICALS 259 GENERAL INDEX 263 V SIMPLE CHEMICAL EXPERIMENTS *> CHAPTER I I YOUR LABORATORY I OST of the experiments described in this book can be M performed without elaborate equipment or apparatus. | For them you will need only a few bottles, test-tubes, meas- i uring-spoons, and an alcohol lamp. Jelly glasses, mayonnaise f jars, small enameled saucepans, and thin glass tumblers can | often be substituted for the beakers, flasks, and glassware of I the professional chemist. t A few of the experiments require beakers, flasks, tubing, | funnels, filter paper, crucibles, mortar and pestle, and Bunsen I burner. The small sizes of these are not expensive. Frequently the cost of apparatus and chemicals can be shared by estab lishing a "community" laboratory which is used by two or more experimenters. -

How to Handle Cryogenic Liquids
Photographic Processing Hazards, by Michael McCann, Ph.D., CIH • Black-and-White Photographic Processing o Mixing Photochemicals o Developing Baths o Stop Baths and Fixers o Intensifiers and Reducers o Toners o Other Hazards • Color Processing o Developing Baths o Bleaching, Fixing, and Other Steps Black-and-White Photographic Processing A wide variety of chemicals are used in black and white photographic processing. Film developing is usually done in closed canisters. Print processing uses tray processing, with successive developing baths, stop baths, fixing baths, and rinse steps. Other treatments include use of hardeners, intensifiers, reducers, toners, and hypo eliminators. Mixing Photochemicals Photochemicals can be bought in liquid form, which only need diluting, or powder form, which need dissolving and diluting. Hazards 1. Developer solutions and powders are often highly alkaline, and glacial acetic acid, used in making the stop bath, is also corrosive by skin contact, inhalation and ingestion. 2. Developer powders are highly toxic by inhalation, and moderately toxic by skin contact, due to the alkali and developers themselves (see Developing Baths below). Precautions 1. Use liquid chemistry whenever possible, rather than mixing developing powders. Pregnant women, in particular, should not be exposed to powdered developer. 2. When mixing powdered developers, use a glove box (a cardboard box with glass or plexiglas top, and two holes in the sides for hands and arms), local exhaust ventilation, or wear a NIOSH-approved toxic dust respirator. 3. Wear gloves, goggles and protective apron when mixing concentrated photochemicals. Always add any acid to water, never the reverse. 4. In case of skin contact, rinse with lots of water. -

Watercolours: What´S Different in the New Assortment?
Product information Watercolours HORADAM® watercolours: What´s different in the new assortment? The following colours have got a new name: Art.-No. Old Name New Name 14 208 Aureolin modern Aureolin hue 14 209 Translucent yellow Transparent Yellow 14 211 Chrome yellow lemon Chromium yellow hue lemon 14 212 Chrome yellow light Chromium yellow hue light 14 213 Chrome yellow deep Chromium yellow hue deep 14 214 Chrome orange Chromium orange hue 14 218 Translucent orange Transparent orange 14 366 Deep red Perylene maroon 14 476 Mauve Schmincke violet 14 498 Dark blue indigo Dark blue 14 648 Sepia brown tone Sepia brown reddish 14 786 Charcoal grey Anthracite The following colours are completely omitted due to raw materials which aren’t available anymore: Art.-No. Old Name 14 652 Walnut brown This tone cannot be mixed out of other colours. 14 666 Pozzuoli earth This tone can be mixed using 14 649 English Venetian red and the slightly reddish 14 670 Madder brown. These colours are now produced using other pigments. Therefore they have got a new name and a new number: Old Art.-No. Old Name New Art.-No. New Name 14 345 Dark red 14 344 Perylene dark red 14 210 Gamboge gum modern 14 217 Quinacridone gold hue 14 478 Helio blue reddish 14 477 Phthalo sapphire blue 14 536 Green yellow 14 537 Transparent green gold - Discontinued colours The described product attributes and application examples have been tes- a warranty for product attributes and/or assume liability for damages that ted in the Schmincke laboratory. -

United States Patent Office Patented Feb
3,234, 66 United States Patent Office Patented Feb. 8, 1966 2 The following example serves to illustrate the values 3,234,166 obtained when the present invention is not followed, all BENZENE-S8:UBLE AND BENZENENSOLUBLE parts being by weight unless otherwise identified. CiS-14 POLYISOPRENE Kenneth (C. iiecker, Aron, Ohio, assignor to The Good Example l-Control year Tire & Ruber Company, Aron, Ohio, a corpora A latex is made by first making a gel-free 96% cis-1,4 ties of binic polyisoprene cement in benzene containing 10% solids Ng Drawing. Fied Ar. 4, 1962, Ser. No. 84,953 and then adding to the cement 5.5 parts of potassium Claira. (C. 260-29.7) oleate, 0.2 parts of the tetra sodium salt of ethylene di amine tetra acetic acid (Versene), the amount of each This invention relates to an improved latex of a solu O material being based on 100 parts of rubber. This mix tion-polymerized diene rubber, a process for its manu ture is then vigorously stirred into 1000 parts of distilled facture and uses thereof. Water to bring about the complete emulsification of the A gum rabber film laid down from a man-made rubber rubber cement in the water. latex and particularly from a latex made from a benzene The benzene is stripped from the emulsion using a soluble polydiene and particularly polyisoprene contain laboratory size disc evaporator which operates by revolv ing at least 50% of cis-1,4 polyisoprene is deficient in ing metal discs through the emulsion, exposing new sur certain physical properties and particularly with respect faces to the atmosphere and thus evaporating the benzene. -

RAL COLOR CHART ***** This Chart Is to Be Used As a Guide Only. Colors May Appear Slightly Different ***** Green Beige Purple V
RAL COLOR CHART ***** This Chart is to be used as a guide only. Colors May Appear Slightly Different ***** RAL 1000 Green Beige RAL 4007 Purple Violet RAL 7008 Khaki Grey RAL 4008 RAL 7009 RAL 1001 Beige Signal Violet Green Grey Tarpaulin RAL 1002 Sand Yellow RAL 4009 Pastel Violet RAL 7010 Grey RAL 1003 Signal Yellow RAL 5000 Violet Blue RAL 7011 Iron Grey RAL 1004 Golden Yellow RAL 5001 Green Blue RAL 7012 Basalt Grey Ultramarine RAL 1005 Honey Yellow RAL 5002 RAL 7013 Brown Grey Blue RAL 1006 Maize Yellow RAL 5003 Saphire Blue RAL 7015 Slate Grey Anthracite RAL 1007 Chrome Yellow RAL 5004 Black Blue RAL 7016 Grey RAL 1011 Brown Beige RAL 5005 Signal Blue RAL 7021 Black Grey RAL 1012 Lemon Yellow RAL 5007 Brillant Blue RAL 7022 Umbra Grey Concrete RAL 1013 Oyster White RAL 5008 Grey Blue RAL 7023 Grey Graphite RAL 1014 Ivory RAL 5009 Azure Blue RAL 7024 Grey Granite RAL 1015 Light Ivory RAL 5010 Gentian Blue RAL 7026 Grey RAL 1016 Sulfer Yellow RAL 5011 Steel Blue RAL 7030 Stone Grey RAL 1017 Saffron Yellow RAL 5012 Light Blue RAL 7031 Blue Grey RAL 1018 Zinc Yellow RAL 5013 Cobolt Blue RAL 7032 Pebble Grey Cement RAL 1019 Grey Beige RAL 5014 Pigieon Blue RAL 7033 Grey RAL 1020 Olive Yellow RAL 5015 Sky Blue RAL 7034 Yellow Grey RAL 1021 Rape Yellow RAL 5017 Traffic Blue RAL 7035 Light Grey Platinum RAL 1023 Traffic Yellow RAL 5018 Turquiose Blue RAL 7036 Grey RAL 1024 Ochre Yellow RAL 5019 Capri Blue RAL 7037 Dusty Grey RAL 1027 Curry RAL 5020 Ocean Blue RAL 7038 Agate Grey RAL 1028 Melon Yellow RAL 5021 Water Blue RAL 7039 Quartz Grey -

1 Abietic Acid R Abrasive Silica for Polishing DR Acenaphthene M (LC
1 abietic acid R abrasive silica for polishing DR acenaphthene M (LC) acenaphthene quinone R acenaphthylene R acetal (see 1,1-diethoxyethane) acetaldehyde M (FC) acetaldehyde-d (CH3CDO) R acetaldehyde dimethyl acetal CH acetaldoxime R acetamide M (LC) acetamidinium chloride R acetamidoacrylic acid 2- NB acetamidobenzaldehyde p- R acetamidobenzenesulfonyl chloride 4- R acetamidodeoxythioglucopyranose triacetate 2- -2- -1- -β-D- 3,4,6- AB acetamidomethylthiazole 2- -4- PB acetanilide M (LC) acetazolamide R acetdimethylamide see dimethylacetamide, N,N- acethydrazide R acetic acid M (solv) acetic anhydride M (FC) acetmethylamide see methylacetamide, N- acetoacetamide R acetoacetanilide R acetoacetic acid, lithium salt R acetobromoglucose -α-D- NB acetohydroxamic acid R acetoin R acetol (hydroxyacetone) R acetonaphthalide (α)R acetone M (solv) acetone ,A.R. M (solv) acetone-d6 RM acetone cyanohydrin R acetonedicarboxylic acid ,dimethyl ester R acetonedicarboxylic acid -1,3- R acetone dimethyl acetal see dimethoxypropane 2,2- acetonitrile M (solv) acetonitrile-d3 RM acetonylacetone see hexanedione 2,5- acetonylbenzylhydroxycoumarin (3-(α- -4- R acetophenone M (LC) acetophenone oxime R acetophenone trimethylsilyl enol ether see phenyltrimethylsilyl... acetoxyacetone (oxopropyl acetate 2-) R acetoxybenzoic acid 4- DS acetoxynaphthoic acid 6- -2- R 2 acetylacetaldehyde dimethylacetal R acetylacetone (pentanedione -2,4-) M (C) acetylbenzonitrile p- R acetylbiphenyl 4- see phenylacetophenone, p- acetyl bromide M (FC) acetylbromothiophene 2- -5- -

Asphalt-Rubber Interactions
TRANSPORTATION RESEARCH RECORD 1417 99 Asphalt-Rubber Interactions MARY STROUP-GARDINER, DAVIDE. NEWCOMB, AND BRUCE TANQUIST Three experiments were designed to evaluate the influence of as the wet process and adds the rubber to the binder. With inc~easing percentages of rubber (0, 10, 15, and 20 percent by sufficient time and heat, a partially polymer modified asphalt weight of bmder) and rubber type (passenger and industrial tires), cement is achieved as the rubber is slowly depolymerized pretreatment of rubber (none, tall oil pitch), and asphalt chem (J ,p.203). When the wet process is used, a high degree of istry (Strategic Highway Research Program materials reference library asphalts AAD, AAG, AAF, and AAM) on asphalt interaction between the asphalt and the rubber is desired to rubber interactions. Viscosity was measured by a Brookfield ro accelerate the depolymerization of the rubber particles (J). tational viscometer; testing variability was also estimated. Un The second method, the dry process, uses the rubber as an treated rubber was included to represent interaction character aggregate replacement. Although some reaction of the rubber istics representative of the wet process. Treated rubber was included with the asphalt occurs, the primary goal of this approach is as a way of stabilizing the rubber before use in the dry process. to provide solid elastomeric inclusions within the asphalt Results confjrmed_ that.rubber (either passenger or industrial tires) aggregate matrix. These inclusions are thought to provide re~cts more readily with a softer grade of asphalt from a given refinery. Pretreat~e~t of ~as~e.nger tire rubber with various per more rebound to the mixture under traffic loading. -

Repetitive Use Injuries
PATIENT HANDOUT Repetitive Use Injuries as assembly-line workers, stock clerks, ware- enjoy doing. Here are six simple hand exercises house workers, transcriptionists and garment that will move your joints, stretch your tendons workers are at risk. Also, people who work as and keep your hands healthy: gardeners, bank tellers, musicians and even athletes should be aware of RSIs. 1. Place both hands in front of you and stretch your fingers out, keeping them up and open DO I HAVE AN RSI? for a few seconds. These injuries are more than just a sore wrist or aching fingers. Overuse and misuse of the 2. Keep both of your hands in front of you and hand can lead to serious injuries. You should curl your fingers and thumb into a ball.H old be aware of the following injuries and take pre- this position a few seconds. ventative measure to avoid them: 3. Put your arms in front of you and lift your Carpal Tunnel Syndrome: This compression right hand so that the palm of your hand of the median nerve through the carpal tunnel, points out and your fingers face up. Using located at the base of the hand, causes numbness your other hand, push the fingers towards e take medicine to improve blood and tingling of the fingers, weakness and pain. your body until you feel a slight stretch. pressure. We try to eat foods that Repeat with the other hand. will decrease our cholesterol. DeQuervain’s Tenosynovitis: This RSI is local- And we exercise to keep our ized to the tendons of the thumb at the level of 4.