MVA85A Efficacy Trials: First Results in African Infants
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The 6-In-1 Vaccine Study
Oxford Vaccine Group University of Oxford Centre for Clinical Vaccinology and Tropical Medicine, Churchill Hospital, Headington, Oxford OX3 7LE Telephone: 01865 611400 [email protected] www.ovg.ox.ac.uk The 6-in-1 vaccine study Study Information Booklet We are inviting infants aged 8-13 weeks of age to take part in a study comparing two licensed combination vaccines that can be given in the routine UK immunisation schedule. These vaccines protect against 6 different infections in the one injection. Before you decide to take part in this study, it is important for you to understand what the study is about and what participation would involve. Please take time to read the information carefully, and discuss with others if you wish. If you have any questions please contact the study team. Thank you for taking the time to consider this study. Contact the local study team at: Oxford Vaccine Group CCVTM, Churchill Hospital, Oxford, OX3 7LE 01865 611400 [email protected] www.ovg.ox.ac.uk/recruiting-studies Page 1 of 11 The 6-in-1 vaccine study; OVG 2018/05; Participant Information Booklet; REC Ref: 19/SC/0052; IRAS ID: 252324; Version 2.0 dated 23-APR-2019 Oxford Vaccine Group University of Oxford Centre for Clinical Vaccinology and Tropical Medicine, Churchill Hospital, Headington, Oxford OX3 7LE Telephone: 01865 611400 [email protected] www.ovg.ox.ac.uk Summary • This study will help us to better understand the interaction between ‘6- in-1’ vaccines and the Meningococcal B vaccine in the routine UK immunisation schedule • We will be enrolling 240 healthy babies aged 8 – 13 weeks into this study • Babies will receive all their infant immunisations in their own home or in a convenient location until 12 months of age • There will be six study visits including 4 vaccine visits and 2 visits for blood sampling (we will use a topical anaesthetic cream to numb the skin for blood sampling) Why has my child been invited to take part? You have been approached as your child is in the age range for this study and lives in the Thames Valley. -
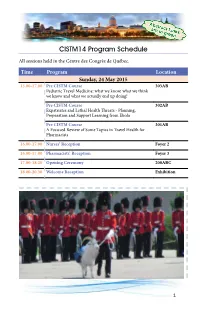
CISTM14 Program Schedule
CISTM14 Program Schedule All sessions held in the Centre des Congrès de Québec. Time Program Location Sunday, 24 May 2015 13.00-17.00 Pre CISTM Course 303AB Pediatric Travel Medicine: what we know, what we think we know and what we actually end up doing! Pre CISTM Course 302AB Expatriates and Lethal Health Threats - Planning, Preparation and Support Learning from Ebola Pre CISTM Course 301AB A Focused Review of Some Topics in Travel Health for Pharmacists 16.00-17.00 Nurses’ Reception Foyer 2 16.00-17.00 Pharmacists’ Reception Foyer 3 17.00-18.20 Opening Ceremony 200ABC 18.00-20.30 Welcome Reception Exhibition 1 Time Program Location Monday, 25 May 2015 MTH1 Meet The History 301AB 8.00-8.45 History of the Quarantine Station at Grosse Ile Marc Desmeules, Canada 8.00-8.45 CDC Yellow Book 302AB Gary Brunette, United States of America COD1 Case of the Day 303AB 8.00-8.45 Pre-Travel Rogelio Lopez-Velez, Spain PL1 Plenary 200ABC 9.00-10.30 Our Shrinking World: Health in the 21st Century Chairs: David R. Shlim, United States of America Leo Visser, The Netherlands PL1.01 Measuring global health: The global is local [Change your mindset! It’s time for a reality check] Louis Loutan, Switzerland • Outline the most important trends and health challenges in the world using gapminder/health metrics • Appraise how these changes (global health transitions, global population growth, urbanization, changes in animal helath and human-animal population interactions, and climate change) will influence global migration and travel medicine now and in the future • Integrate global health concepts into thinking about travel medicine 2 Time Program Location Monday, 25 May 2015, continued Cont. -

Sir Bryn Terfel Premieres John Rutter's 'Joseph's Carol', Dedicated to the Oxford Vaccine Team in Celebratory Concert Fr
Sir Bryn Terfel premieres John Rutter’s ‘Joseph’s Carol’, dedicated to the Oxford vaccine team in celebratory concert from the Oxford Philharmonic Orchestra Friday 18 December 2020, 18:30 Streamed on Oxford Philharmonic Orchestra’s YouTube Channel: bit.ly/OPOVaccineTribute Elgar Chanson de Matin William Henry Monk Abide with Me Rodgers & Hammerstein You’ll Never Walk Alone John Rutter Joseph’s Carol WORLD PREMIERE John Rutter Look to the Day Handel Hallelujah Chorus Sir Bryn Terfel bass-baritone Oxford Philharmonic Orchestra Maxim Vengerov violin Choir of Merton College, Oxford John Rutter conductor Alexandra Lowe soprano Marios Papadopoulos conductor Alexander Olleson treble John Suchet presenter In recognition of the formidable work accomplished by the team of scientists at the University of Oxford on their Covid-19 vaccine, the Oxford Philharmonic Orchestra will stream a celebratory concert on Friday 18 December, recorded in the city’s historic Sheldonian Theatre. Performed by bass-baritone Sir Bryn Terfel, the short concert features the premiere of John Rutter’s Joseph’s Carol, written in tribute to the Oxford Vaccine Group, the Jenner Institute and the RECOVERY team. The words by John Rutter recount the long and weary journey of Joseph and Mary to Bethlehem before the birth of the baby Jesus, echoing the programme’s journey from struggle through to hope. Bryn Terfel also joins the Orchestra and the Choir of Merton College, Oxford, in a rousing programme from Rodgers & Hammerstein’s You’ll Never Walk Alone (with Jette Parker Young Artist Alexandra Lowe) to Handel’s Hallelujah Chorus. Sir Bryn and the Orchestra are also joined in the hymn of comfort, Abide with Me, by chorister Alexander Olleson of Christ Church Cathedral Choir, the recent winner of BBC Young Chorister Of The Year 2020. -

Download a PDF of Our Community Brochure
Engagement with the communities of Oxford and Oxfordshire Did you know? St Giles’ Fair began as the parish feast of St Giles, first recorded in 1624. From the 1780s it became a toy fair, with general amusements for children. In the next century its focus shifted towards adults, with entertainment, rides and stalls. In the late 1800s there were calls for the fair to be stopped on the grounds that it encouraged rowdy behaviour. During Victorian times engineering advances brought the forerunners of today’s rides. Today the huge pieces of machinery fill St Giles’ with sparkling lights for a few days each year, and whizz within feet of ancient college buildings. The stone heads around the Sheldonian Theatre now number thirteen (there were originally fourteen, but one was removed to make way for the adjoining Clarendon Building.) It is not known what they were intended to represent – they might be gods, wise men, emperors or just boundary markers. The original heads were made by William Byrd and put up in 1669. Did you Replacements put up in 1868 were made in poor stone, know? which crumbled away; in 1972 the current set, carved by Michael Black of Oxford, were erected. More on page 4 STARGAZING AND SPIN-OUTS PAGE 1 Contents 2 Introduction from the Vice-Chancellor 3 Foreword from the Chair of the Community Engagement Group 5 Part 1: Part of the fabric of the city Part of the fabric 6 800 years of history of the 8 Economic impact city 9 Science Parks 1 0 Saïd Business School 11 Oxford University Press PART 1 PART 1 2 The built environment 13 -

RCN International Nursing Research Conference 2017
RCN International Nursing Research Conference 2017 Wednesday 5 – Friday 7 April 2017 University of Oxford Examination Schools, 75-81 High Street, Oxford, OX1 4BG, UK Conference abstracts media partner Accrue up to 27 hours #research2017 of CPD Contents Keynote speaker abstracts 4 Concurrent session 6 54 Thursday 6 April 2017 2-2.55pm 54 Theme: Focus groups .................................54 Concurrent session 1 6 Theme: Mixed ........................................55 Wednesday 5 April 2017 11.30am-12.55pm 6 Theme: Qualitative approaches/patient safety and experience .....................................56 Theme: Qualitative approaches ..........................6 Theme: Qualitative approaches/interviews ...............57 Theme: Qualitative approaches .......................... 7 Theme: Evidence review ...............................58 Theme: Qualitative approaches ..........................8 Theme: Qualitative approaches/text and discourse ........59 Theme: Evidence review/patient safety ..................10 Theme: Questionnaires/other methods ..................60 Theme: Mixed eHealth .................................11 Theme: Research methodology ......................... 13 Concurrent session 7 62 Theme: Mixed methods/patient experience ............... 14 Friday 7 April 2017 9.50-10.45am 62 Concurrent session 2 17 Theme: Workforce/review .............................62 Wednesday 5 April 2017 1.55-3.20pm 17 Theme: Qualitative approaches .........................63 Theme: Qualitative approaches/interviewing .............64 Theme: Qualitative -

'Astrazeneca' Covid-19 Vaccine
Medicines Law & Policy How the ‘Oxford’ Covid-19 vaccine became the ‘AstraZeneca’ Covid-19 vaccine By Christopher Garrison 1. Introduction. The ‘Oxford / AstraZeneca’ vaccine is one of the world’s leading hopes in the race to end the Covid-19 pandemic. Its history is not as clear, though, as it may first seem. The media reporting about the vaccine tends to focus either on the very small (non-profit, academic) Jenner Institute at Oxford University, where the vaccine was first invented, or the very large (‘Big Pharma’ firm) AstraZeneca, which is now responsible for organising its (non-profit) world-wide development, manufacture and distribution. However, examining the intellectual property (IP) path of the vaccine from invention to manufacture and distribution reveals a more complex picture that involves other important actors (with for-profit perspectives). Mindful of the very large sums of public money being used to support Covid-19 vaccine development, section 2 of this note will therefore contextualise the respective roles of the Jenner Institute, AstraZeneca and these other actors, so that their share of risk and (potential) reward in the project can be better understood. Section 3 provides comments as well as raising some important questions about what might yet be done better and what lessons can be learned for the future. 2. History of the ‘Oxford / AstraZeneca’ vaccine. 2.1 Oxford University and Oxford University Innovation Ltd. The Bayh-Dole Act (1980) was hugely influential in the United States and elsewhere in encouraging universities to commercially exploit the IP they were generating by setting up ‘technology transfer’ offices. -

Job Description and Person Specificationselection Criteria
Job title Senior Postdoctoral Scientist Division Medical Sciences Department Nuffield Department of Medicine, Jenner Institute Clinical Centre for Vaccinology and Translational Medicine Location (CCVTM), Old Road Campus, Headington, Oxford, OX3 7LE Grade 8: £41,526 - £49,553 per annum with a discretionary range Grade and salary to £54,131 per annum Hours Full time Contract type Fixed-term contract for 20 months, in the first instance Reporting to Professor Christine Rollier & Professor Cal MacLennan Vacancy reference 147960 Additional Position funded by US funding information Development of a Gonococcal Outer Membrane Vesicle Vaccine Research topic from Lead Optimization to Phase 1 Clinical Trial Principal Investigator Professor Christine Rollier & Professor Cal MacLennan / supervisor Project team Gonococcal Vaccine Group http://www.jenner.ac.uk/ Project web site Funding partner US funding 1. Gottlieb SL, et al. Gonococcal vaccines: public health value and preferred product characteristics; report of a WHO global stakeholder consultation, January 2019. Vaccine 2020; 38: 4362-4373. 2. Micoli, F et al. Comparative immunogenicity and efficacy of equivalent outer membrane vesicle and glycoconjugate vaccines against nontyphoidal Salmonella. PNAS 2018; Recent publications 115: 10428-33. 3. Folegatti PM, et al. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: a preliminary report of a phase 1/2, single-blind, randomised controlled trial. Lancet 2020; 396: 467-478. 4. Marsay L, et al. A novel meningococcal outer membrane vesicle vaccine with constitutive expression of FetA: A phase I clinical trial. J Infect 2015; 71: 326-37. The Role The Gonococcal Vaccine Project is based at the Jenner Institute, University of Oxford, and utilizes a novel outer membrane vesicle (OMV) technology to develop a vaccine against gonorrhoea. -

Revised Register of Congregation Qualified Under the Provisions of Sect 3, Stat IV, of the Statutes of the University
WEDNESDay 4 fEbruary 2015 • SuPPLEMENT (1) TO NO 5084 • VOL 145 Gazette Supplement Revised Register of Congregation Qualified under the provisions of Sect 3, Stat IV, of the Statutes of the University aarnio, O M, St Edmund Hall aldridge, H D J N, Development Office andrews, K S, Development Office aarts, D G a L, Christ Church aldridge, S, Queen's andrews, L a, research Services abate, a, Linacre aleksidze, N, Pembroke andrews, T M, faculty of Clinical Medicine abecassis, M, Wadham alen amaro, C M, Linacre andreyev, C C L, Christ Church abeler, J, St anne's alexander, C a H, Merton angel, a G, Dept of biochemistry aboobaker, a, Lady Margaret Hall alexander, J H, Linacre angelou, C L, Development Office abouzayd, S, Christ Church ali, H, faculty of Clinical Medicine angiolini, Dame Elish, St Hugh's abraham, D b, Wolfson ali, r, Cancer research uK Epidemiology unit angus, b J, NDM Experimental Medicine abrams, L J, balliol alkire, S M, Magdalen Division abramsky, S, Wolfson allan, C L, Wolfson anonuevo, E, finance Division acheson, D J, Jesus allan, J M, bodleian Enterprise units ansell, b W, Nuffield ackermann, S M, Linacre allan, J W, St Cross ansorge, O, Christ Church ackroyd, J M a, Social Sciences Division allan, W, university College anthony, D C, Somerville acuto, O, Lincoln allen, a M, Exeter antonini, M, St Peter's adam, a K M, St Stephen's House allen, C a, St Catherine's antoranz Contera, S, Green Templeton adam, C S, St Cross allen, G f, Pembroke aoki, y, Dept of Physiology, anatomy and adams, a C G, Merton allen, J M W, St -

Title in Here, Arial 14 Bold, Caps
News Release AstraZeneca and Oxford University announce landmark agreement for COVID-19 vaccine Collaboration will enable global development, manufacturing and distribution of the vaccine 30 April 2020 AstraZeneca and the University of Oxford today announced an agreement for the global development and distribution of the University’s potential recombinant adenovirus vaccine aimed at preventing COVID-19 infection from SARS-CoV-2. The collaboration aims to bring to patients the potential vaccine known as ChAdOx1 nCoV- 19, being developed by the Jenner Institute and Oxford Vaccine Group, at the University of Oxford. Under the agreement, AstraZeneca would be responsible for development and worldwide manufacturing and distribution of the vaccine. Pascal Soriot, Chief Executive Officer, AstraZeneca, said: “As COVID-19 continues its grip on the world, the need for a vaccine to defeat the virus is urgent. This collaboration brings together the University of Oxford’s world-class expertise in vaccinology and AstraZeneca’s global development, manufacturing and distribution capabilities. Our hope is that, by joining forces, we can accelerate the globalisation of a vaccine to combat the virus and protect people from the deadliest pandemic in a generation.” Mene Pangalos, Executive Vice President, BioPharmaceuticals R&D, AstraZeneca, said: “The University of Oxford and AstraZeneca have a longstanding relationship to advance basic research and we are hugely excited to be working with them on advancing a vaccine to prevent COVID-19 around the world. We are looking forward to working with the University of Oxford and innovative companies such as Vaccitech, as part of our new partnership.” Alok Sharma, UK Business Secretary, said: “This collaboration between Oxford University and AstraZeneca is a vital step that could help rapidly advance the manufacture of a coronavirus vaccine. -

Revised Register of Congregation Qualified Under the Provisions of Sect 3, Stat IV, of the Statutes of the University
WEDNESDAY 4 MARCH 2020 • SUPPLEMENT (1) TO NO 5268 • VOL 150 Gazette Supplement Revised Register of Congregation Qualified under the provisions of Sect 3, Stat IV, of the Statutes of the University Aarnio, O M, St Edmund Hall Ahearn-Ligham, A, Green Templeton Allen, W L, Magdalen Aarts, D G A L, Christ Church Ahern, G, IT Services Allendorf, K I, Corpus Christi Abate, A, Linacre Ahmed, A A, St Hugh's Allison, J R, Christ Church Abbasi, S, Kellogg Ahmed, B, Department of Physiology, Allison, R A, St Antony's Abdou, A M A, Oriental Studies Anatomy, and Genetics Al-Mossawi, M H, St Edmund Hall Abecassis, M, Wadham Ahmed, F, Faculty of Clinical Medicine Alsop, P J, Wadham Abeler, J, St Anne's Ahmed, I, Department of Earth Sciences Altehenger, J E, Merton Abeler-Dorner, L E E, CR Big Data Institute Aigrain, S, All Souls Altmann, P, Faculty of Clinical Medicine Building Ainsworth, T R, Trinity Alvand, A, Wolfson Abell, C E J, Queen's Airoldi, M, Green Templeton Alvergne, A E, Harris Manchester Aboobaker, A, Lady Margaret Hall Aitken, E M, IT Services Amel-Zadeh, A, Green Templeton Abouzayd, S, Christ Church Aitken, G, St Hugh's Amengual, M R, Kellogg Abrams, L J, Balliol Akam, T E, Worcester Anand, G, Department of Paediatrics Abramsky, S, Wolfson Akande, D O, Exeter Anastasakis, O, St Antony's Abulafia, A B S, Lady Margaret Hall Akbar, N, RDM Department of Cardiovascular Anderson, B J, Wadham Acedo-Matellán, V, Oriel Medicine Anderson, E A, Jesus Acharya, D N, All Souls Akerman, C J, Corpus Christi Anderson, H L, Keble Achtnich, M, Magdalen -

Newsletter 31: Spring 2014
Welcome to the spring edition of the VACCSline newsletter This edition of the newsletter focuses on some of the questions that were submitted by delegates attending the Oxford Vaccine Group Immunisation seminar on the 21st March for the panel to answer. Boosters of Meningococcal C vaccine Question: Should 13/14 year olds who present at their GP surgery be vaccinated by the practice nurse or referred back to the school nursing services for vaccination? Answer: The adolescent booster of MenC has been commissioned to be delivered by the school health nursing (SHN) service. However there is an expectation that GP practices will vaccinate a small minority of teenagers presenting in primary care for a valid reason, for example, true needle phobias. SHN teams are expected to make formal referrals for such cases. All other provision should be via school health nursing. Question: Are we meant to be boosting all 20-25 year olds with MenC vaccine? Answer: No. If an individual up to the age of 25 years has received no MenC vaccine previously then they should receive one dose, but boosting is not routine unless part of the university programme (see below). Further details of the MenC vaccination schedule for those with unknown or incomplete vaccination histories can be found in table 22.2 within the Meningococcal chapter of the online green book accessed at: https://www.gov.uk/government/collections/immunisation-against-infectious-disease-the-green-book Question: Should we be offering MenC vaccine to individuals going off to university? Answer: The programme for new starters at university to receive a single dose of MenC is due to start from late summer 2014. -

Reunification
THE OLD OUNDELIAN 2019-2020 Iain Farrington Brian Hemingway REUNIFICATION ( C 95 ) on pop ( St A 70 ) writes On the tenth anniversary of the formal and jazz, Horrible about his father, unification of Oundle School and Laxton Histories, and Paddy, the last School, Philip Sloan ( LS 71 ) gives a making classical surviving Battle of historical perspective music accessible to all Britain fighter pilot OFFICERS The Old Oundelian Club PRESIDENT: Charles Miller ( Ldr 76 ) SECRETARY, TREASURER AND LONDON DINNER SECRETARY: Jane Fenton ADDRESS: The Stables, Cobthorne, West Street, Oundle PE8 4EF. TEL: 01832 277297. EMAIL: [email protected] VICE PRESIDENTS Mary Price ( K 94 ) Hon Sec OO Women’s Hockey Alastair Irvine ( Sc 81 ) Nina Rieck ( K 95 ) Alice Rockall ( W 12 ) Chris Piper ( Sc 71 ) Email: [email protected] SPORTS SECRETARIES LIFE VICE PRESIDENTS Hon Sec Oundle Rovers CC Hon Sec Netball Nick Cheatle ( G 63 ) Chris Piper ( Sc 71 ) Rachel Hawkesford ( W 08 ) John Crabbe ( G 55 ) Email: [email protected] Email: [email protected] Shane Dodd ( Sn 74 ) Hon Sec OORUFC Robert Ellis ( D 65 ) Hon Sec OO Rifle Club Sam Cone ( St A 05 ) Sir Michael Pickard ( C 51 ) Charles Shelley ( S 18 ) Email: [email protected] Chris Piper ( Sc 71 ) Email: [email protected] Chris Walliker ( D 54 ) Hon Sec OO Golfing Society Hon Sec OO Rowing Club Harry Williamson ( St A 55 ) James Aston ( St A 92 ) Kristina Cowley ( L 13 ) Email: [email protected] FINANCE AND POLICY COMMITTEE Email: [email protected] Alastair Irvine ( Sc