Global Change Impacts and Conservation Priorities in the Iberian Peninsula
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

New Data on the Chewing Lice (Phthiraptera) of Passerine Birds in East of Iran
See discussions, stats, and author profiles for this publication at: https://www.researchgate.net/publication/244484149 New data on the chewing lice (Phthiraptera) of passerine birds in East of Iran ARTICLE · JANUARY 2013 CITATIONS READS 2 142 4 AUTHORS: Behnoush Moodi Mansour Aliabadian Ferdowsi University Of Mashhad Ferdowsi University Of Mashhad 3 PUBLICATIONS 2 CITATIONS 110 PUBLICATIONS 393 CITATIONS SEE PROFILE SEE PROFILE Ali Moshaverinia Omid Mirshamsi Ferdowsi University Of Mashhad Ferdowsi University Of Mashhad 10 PUBLICATIONS 17 CITATIONS 54 PUBLICATIONS 152 CITATIONS SEE PROFILE SEE PROFILE Available from: Omid Mirshamsi Retrieved on: 05 April 2016 Sci Parasitol 14(2):63-68, June 2013 ISSN 1582-1366 ORIGINAL RESEARCH ARTICLE New data on the chewing lice (Phthiraptera) of passerine birds in East of Iran Behnoush Moodi 1, Mansour Aliabadian 1, Ali Moshaverinia 2, Omid Mirshamsi Kakhki 1 1 – Ferdowsi University of Mashhad, Faculty of Sciences, Department of Biology, Iran. 2 – Ferdowsi University of Mashhad, Faculty of Veterinary Medicine, Department of Pathobiology, Iran. Correspondence: Tel. 00985118803786, Fax 00985118763852, E-mail [email protected] Abstract. Lice (Insecta, Phthiraptera) are permanent ectoparasites of birds and mammals. Despite having a rich avifauna in Iran, limited number of studies have been conducted on lice fauna of wild birds in this region. This study was carried out to identify lice species of passerine birds in East of Iran. A total of 106 passerine birds of 37 species were captured. Their bodies were examined for lice infestation. Fifty two birds (49.05%) of 106 captured birds were infested. Overall 465 lice were collected from infested birds and 11 lice species were identified as follow: Brueelia chayanh on Common Myna (Acridotheres tristis), B. -

5.4. Changes in the Bird Communities of Sierra Nevada Zamora ,R.1 and Barea-Azcón, J.M.2 1 Andalusian Institute for Earth System Research
5.4. Changes in the bird communities of Sierra Nevada Zamora ,R.1 and Barea-Azcón, J.M.2 1 Andalusian Institute for Earth System Research. University of Granada 2 Environment and Water Agency of Andalusia Abstract The changes in the composition and abundance of passerine communities were studied along an elevational gradient, comparing the results found by censuses made in three different habitats (oak forest, high-mountain juniper scrublands, and high-mountain summits) at the beginning of the 1980s and at present. The results indicate that in the last 30 years, notable changes have taken place in the composition and, especially, in the abundance of the passerine communities. Significant declines in populations were appreciated in many of the species that were dominant in the 1980s, particularly in oak forests and in high-mountain juniper scrublands. The magnitude of the changes diminishes with elevation, and therefore the ecosystem that has changed the most was the oak woodland and those that changed the least were the ecosystems of the high-summits. The bird communities in Sierra Nevada showed a strong spatio-temporal dynamic that appears to be accentuated by global change. Aims and methodology The censuses of reproductive birds compiled censuses were made along linear transects with [13 - 17]. The current censuses were undertaken at the beginning of the 1980s and at present a fixed bandwidth of 50 m, 25 m on each side of within the framework of the Sierra Nevada (2008-2012) were compared. The sites studied the observer. The sampling effort was similar in Global Change Observatory from 2008 to 2012, were the same in both periods: an oak forest both periods. -

Hungary & Transylvania
Although we had many exciting birds, the ‘Bird of the trip’ was Wallcreeper in 2015. (János Oláh) HUNGARY & TRANSYLVANIA 14 – 23 MAY 2015 LEADER: JÁNOS OLÁH Central and Eastern Europe has a great variety of bird species including lots of special ones but at the same time also offers a fantastic variety of different habitats and scenery as well as the long and exciting history of the area. Birdquest has operated tours to Hungary since 1991, being one of the few pioneers to enter the eastern block. The tour itinerary has been changed a few times but nowadays the combination of Hungary and Transylvania seems to be a settled and well established one and offers an amazing list of European birds. This tour is a very good introduction to birders visiting Europe for the first time but also offers some difficult-to-see birds for those who birded the continent before. We had several tour highlights on this recent tour but certainly the displaying Great Bustards, a majestic pair of Eastern Imperial Eagle, the mighty Saker, the handsome Red-footed Falcon, a hunting Peregrine, the shy Capercaillie, the elusive Little Crake and Corncrake, the enigmatic Ural Owl, the declining White-backed Woodpecker, the skulking River and Barred Warblers, a rare Sombre Tit, which was a write-in, the fluty Red-breasted and Collared Flycatchers and the stunning Wallcreeper will be long remembered. We recorded a total of 214 species on this short tour, which is a respectable tally for Europe. Amongst these we had 18 species of raptors, 6 species of owls, 9 species of woodpeckers and 15 species of warblers seen! Our mammal highlight was undoubtedly the superb views of Carpathian Brown Bears of which we saw ten on a single afternoon! 1 BirdQuest Tour Report: Hungary & Transylvania 2015 www.birdquest-tours.com We also had a nice overview of the different habitats of a Carpathian transect from the Great Hungarian Plain through the deciduous woodlands of the Carpathian foothills to the higher conifer-covered mountains. -

Belarus in Spring
Belarus in Spring Naturetrek Tour Report 6 - 13 May 2012 Report compiled by Attila Steiner Naturetrek Cheriton Mill Cheriton Alresford Hampshire SO24 0NG England T: +44 (0)1962 733051 F: +44 (0)1962 736426 E: [email protected] W: www.naturetrek.co.uk Tour Report Belarus in Spring Tour leaders: Attila Steiner Alexander Duka Participants: Elizabeth Briggs David Briggs Colin Hughes John Skeavington Jillian Bale Roberta Goodall Day 1 Sunday 6th May UK – Minsk – Liaskavichi Our flight from London arrived on time at Minsk International Airport. At the arrival hall Attila and Alexander greeted us. After changing money we had a tasty dinner at the airport restaurant. Then we started the long drive to our first hotel situated on the edge of the famous Pripiat National Park. We arrived at our hotel in Liaskavichi after midnight. After checking in some of us could hear Thrush Nightingale singing and Spotted Crakes calling from the nearby wetland. Day 2 Monday 7th May Liaskavich area of Pripiat National Park It was already sunny and hot outside when we gathered at the minivan ready to explore nearby woodlands and wetlands. As we drove through Liaskavichi we saw our first White Stork nest, one of the hundreds to be seen during the week. Our first stop along the road was for a raptor circling above the fields, which proved to be a Lesser Spotted Eagle. We soon left the main road and took a gravel road towards the woodlands. We stopped to watch a Black Kite flying above the forest. It then landed on top of a dead tree and we had prolonged scope views of this nice raptor. -
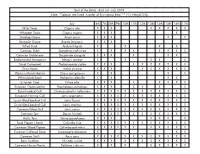
Best of the Baltic - Bird List - July 2019 Note: *Species Are Listed in Order of First Seeing Them ** H = Heard Only
Best of the Baltic - Bird List - July 2019 Note: *Species are listed in order of first seeing them ** H = Heard Only July 6th 7th 8th 9th 10th 11th 12th 13th 14th 15th 16th 17th Mute Swan Cygnus olor X X X X X X X X Whopper Swan Cygnus cygnus X X X X Greylag Goose Anser anser X X X X X Barnacle Goose Branta leucopsis X X X Tufted Duck Aythya fuligula X X X X Common Eider Somateria mollissima X X X X X X X X Common Goldeneye Bucephala clangula X X X X X X Red-breasted Merganser Mergus serrator X X X X X Great Cormorant Phalacrocorax carbo X X X X X X X X X X Grey Heron Ardea cinerea X X X X X X X X X Western Marsh Harrier Circus aeruginosus X X X X White-tailed Eagle Haliaeetus albicilla X X X X Eurasian Coot Fulica atra X X X X X X X X Eurasian Oystercatcher Haematopus ostralegus X X X X X X X Black-headed Gull Chroicocephalus ridibundus X X X X X X X X X X X X European Herring Gull Larus argentatus X X X X X X X X X X X X Lesser Black-backed Gull Larus fuscus X X X X X X X X X X X X Great Black-backed Gull Larus marinus X X X X X X X X X X X X Common/Mew Gull Larus canus X X X X X X X X X X X X Common Tern Sterna hirundo X X X X X X X X X X X X Arctic Tern Sterna paradisaea X X X X X X X Feral Pigeon ( Rock) Columba livia X X X X X X X X X X X X Common Wood Pigeon Columba palumbus X X X X X X X X X X X Eurasian Collared Dove Streptopelia decaocto X X X Common Swift Apus apus X X X X X X X X X X X X Barn Swallow Hirundo rustica X X X X X X X X X X X Common House Martin Delichon urbicum X X X X X X X X White Wagtail Motacilla alba X X -

Multilocus Phylogeny of the Avian Family Alaudidae (Larks) Reveals
1 Multilocus phylogeny of the avian family Alaudidae (larks) 2 reveals complex morphological evolution, non- 3 monophyletic genera and hidden species diversity 4 5 Per Alströma,b,c*, Keith N. Barnesc, Urban Olssond, F. Keith Barkere, Paulette Bloomerf, 6 Aleem Ahmed Khang, Masood Ahmed Qureshig, Alban Guillaumeth, Pierre-André Crocheti, 7 Peter G. Ryanc 8 9 a Key Laboratory of Zoological Systematics and Evolution, Institute of Zoology, Chinese 10 Academy of Sciences, Chaoyang District, Beijing, 100101, P. R. China 11 b Swedish Species Information Centre, Swedish University of Agricultural Sciences, Box 7007, 12 SE-750 07 Uppsala, Sweden 13 c Percy FitzPatrick Institute of African Ornithology, DST/NRF Centre of Excellence, 14 University of Cape Town, Rondebosch 7700, South Africa 15 d Systematics and Biodiversity, Gothenburg University, Department of Zoology, Box 463, SE- 16 405 30 Göteborg, Sweden 17 e Bell Museum of Natural History and Department of Ecology, Evolution and Behavior, 18 University of Minnesota, 1987 Upper Buford Circle, St. Paul, MN 55108, USA 19 f Percy FitzPatrick Institute Centre of Excellence, Department of Genetics, University of 20 Pretoria, Hatfield, 0083, South Africa 21 g Institute of Pure & Applied Biology, Bahauddin Zakariya University, 60800, Multan, 22 Pakistan 23 h Department of Biology, Trent University, DNA Building, Peterborough, ON K9J 7B8, 24 Canada 25 i CEFE/CNRS Campus du CNRS 1919, route de Mende, 34293 Montpellier, France 26 27 * Corresponding author: Key Laboratory of Zoological Systematics and Evolution, Institute of 28 Zoology, Chinese Academy of Sciences, Chaoyang District, Beijing, 100101, P. R. China; E- 29 mail: [email protected] 30 1 31 ABSTRACT 32 The Alaudidae (larks) is a large family of songbirds in the superfamily Sylvioidea. -

Colorado Birds the Colorado Field Ornithologists’ Quarterly
Vol. 50 No. 4 Fall 2016 Colorado Birds The Colorado Field Ornithologists’ Quarterly Stealthy Streptopelias The Hungry Bird—Sun Spiders Separating Brown Creepers Colorado Field Ornithologists PO Box 929, Indian Hills, Colorado 80454 cfobirds.org Colorado Birds (USPS 0446-190) (ISSN 1094-0030) is published quarterly by the Col- orado Field Ornithologists, P.O. Box 929, Indian Hills, CO 80454. Subscriptions are obtained through annual membership dues. Nonprofit postage paid at Louisville, CO. POSTMASTER: Send address changes to Colorado Birds, P.O. Box 929, Indian Hills, CO 80454. Officers and Directors of Colorado Field Ornithologists: Dates indicate end of cur- rent term. An asterisk indicates eligibility for re-election. Terms expire at the annual convention. Officers: President: Doug Faulkner, Arvada, 2017*, [email protected]; Vice Presi- dent: David Gillilan, Littleton, 2017*, [email protected]; Secretary: Chris Owens, Longmont, 2017*, [email protected]; Treasurer: Michael Kiessig, Indian Hills, 2017*, [email protected] Directors: Christy Carello, Golden, 2019; Amber Carver, Littleton, 2018*; Lisa Ed- wards, Palmer Lake, 2017; Ted Floyd, Lafayette, 2017; Gloria Nikolai, Colorado Springs, 2018*; Christian Nunes, Longmont, 2019 Colorado Bird Records Committee: Dates indicate end of current term. An asterisk indicates eligibility to serve another term. Terms expire 12/31. Chair: Mark Peterson, Colorado Springs, 2018*, [email protected] Committee Members: John Drummond, Colorado Springs, 2016; Peter Gent, Boul- der, 2017*; Tony Leukering, Largo, Florida, 2018; Dan Maynard, Denver, 2017*; Bill Schmoker, Longmont, 2016; Kathy Mihm Dunning, Denver, 2018* Past Committee Member: Bill Maynard Colorado Birds Quarterly: Editor: Scott W. Gillihan, [email protected] Staff: Christy Carello, science editor, [email protected]; Debbie Marshall, design and layout, [email protected] Annual Membership Dues (renewable quarterly): General $25; Youth (under 18) $12; Institution $30. -

EUROPEAN BIRDS of CONSERVATION CONCERN Populations, Trends and National Responsibilities
EUROPEAN BIRDS OF CONSERVATION CONCERN Populations, trends and national responsibilities COMPILED BY ANNA STANEVA AND IAN BURFIELD WITH SPONSORSHIP FROM CONTENTS Introduction 4 86 ITALY References 9 89 KOSOVO ALBANIA 10 92 LATVIA ANDORRA 14 95 LIECHTENSTEIN ARMENIA 16 97 LITHUANIA AUSTRIA 19 100 LUXEMBOURG AZERBAIJAN 22 102 MACEDONIA BELARUS 26 105 MALTA BELGIUM 29 107 MOLDOVA BOSNIA AND HERZEGOVINA 32 110 MONTENEGRO BULGARIA 35 113 NETHERLANDS CROATIA 39 116 NORWAY CYPRUS 42 119 POLAND CZECH REPUBLIC 45 122 PORTUGAL DENMARK 48 125 ROMANIA ESTONIA 51 128 RUSSIA BirdLife Europe and Central Asia is a partnership of 48 national conservation organisations and a leader in bird conservation. Our unique local to global FAROE ISLANDS DENMARK 54 132 SERBIA approach enables us to deliver high impact and long term conservation for the beneit of nature and people. BirdLife Europe and Central Asia is one of FINLAND 56 135 SLOVAKIA the six regional secretariats that compose BirdLife International. Based in Brus- sels, it supports the European and Central Asian Partnership and is present FRANCE 60 138 SLOVENIA in 47 countries including all EU Member States. With more than 4,100 staf in Europe, two million members and tens of thousands of skilled volunteers, GEORGIA 64 141 SPAIN BirdLife Europe and Central Asia, together with its national partners, owns or manages more than 6,000 nature sites totaling 320,000 hectares. GERMANY 67 145 SWEDEN GIBRALTAR UNITED KINGDOM 71 148 SWITZERLAND GREECE 72 151 TURKEY GREENLAND DENMARK 76 155 UKRAINE HUNGARY 78 159 UNITED KINGDOM ICELAND 81 162 European population sizes and trends STICHTING BIRDLIFE EUROPE GRATEFULLY ACKNOWLEDGES FINANCIAL SUPPORT FROM THE EUROPEAN COMMISSION. -

Birds of Latvia
Birds of Latvia Checklist with remarks about species occurrence All species known to have occured in Latvia are listed in systematic order (based on Voous sequence) following guidelines of Association of European Rarities Committees. The key to each abbreviated status is as follows. Small letters rather than capitals signify a scarcer distribution - less than 10 records for Latvia until 16.08.2010. The avifauna of Latvia includes a total of 344 species. Place for subspecies or new entries you will find at the end of this list. CB - casual breeder MB - migrant breeder PM - passage migrant RB - resident breeder WV - winter visitor SV - summer visitor V - vagrant E - escaped or recently introduced Name, route, dates etc. Categories used for the national list: Category A - species which has been recorded in an apparently wild state at least once since 1.1.1950 [Majority of the species - are not marked in the categories’ column]. Category B - species which has been recorded in an apparently wild state only between 1800 and 1949 Category C - released or escaped species which has established a self-supporting breeding population in the own country; also birds coming from a category C population of another country (with the species not breeding in the own country). ----------------------------------------- Category D - every species unless it is almost certainly a genuine vagrant (in which case it enters Cat. A), or almost certainly an escape from captivity (Cat. E). Here also these species, which has been found dead at the sea-shore and there is doubt about their death within Latvian territory or beyond. -

Functional Correlation Between Habitat Use and Leg Morphology in Birds (Aves)
Blackwell Science, LtdOxford, UKBIJBiological Journal of the Linnean Society0024-4066The Linnean Society of London, 2003? 2003 79? Original Article HABITAT and LEG MORPHOLOGY IN BIRDS ( AVES )A. ZEFFER ET AL. Biological Journal of the Linnean Society, 2003, 79, 461–484. With 9 figures Functional correlation between habitat use and leg morphology in birds (Aves) ANNA ZEFFER, L. CHRISTOFFER JOHANSSON* and ÅSA MARMEBRO Department of Zoology, Göteborg University, Box 463, SE 405 30 Göteborg, Sweden Received 17 May 2002; accepted for publication 12 December 2002 Many of the morphological features of animals are considered to be adaptations to the habitat that the animals uti- lize. The habitats utilized by birds vary, perhaps more than for any other group of vertebrates. Here, we study pos- sible adaptations in the morphology of the skeletal elements of the hind limbs to the habitat of birds. Measurements of the lengths of the femur, tibiotarsus and tarsometatarsus of 323 bird species from 74 families are used together with body mass data, taken from the literature. The species are separated into six habitat groups on the basis of lit- erature data on leg use. A discriminant analysis of the groups based on leg morphology shows that swimming birds, wading birds and ground living species are more easily identified than other birds. Furthermore, functional predic- tions are made for each group based on ecological and mechanical considerations. The groups were tested for devi- ation from the norm for all birds for three indices of size- and leg-length-independent measures of the bones and for a size-independent-index of leg length. -

Tour Report 3Rd – 9Th October 2020
Autumn in the Cairngorms Naturetrek Tour Report 3rd – 9th October 2020 Crested Tit Pine Marten Red Grouse Red Deer Report and images compiled by Tom Brereton Naturetrek Mingledown Barn Wolf's Lane Chawton Alton Hampshire GU34 3HJ UK T: +44 (0)1962 733051 E: [email protected] W: www.naturetrek.co.uk Tour Report Autumn in the Cairngorms Tour participants: Tom Brereton (Leader) with five Naturetrek clients Day 1 Saturday 3rd October The trip started with the collection of four clients from a dull and rainy Inverness Airport. En route to our afternoon destination, we made a brief stop at Alturie located along the southern margin of the Moray Firth east of Inverness, where there were numerous coastal wildfowl including Wigeon, Teal, Eider and best of all a Slavonian Grebe. A Hooded Crow was also seen. We also stopped at a supermarket nearby to buy some goodies for the trip mainly in the form of drinks for evenings at the guest house. We then drove on to Insh Marhses RSPB where we met the other guest on this holiday. Insh is a wonderful reserve for all forms of wildlife including fungi, and there were several Fly Agaric visible under woodland right by the car park. During the afternoon we made a short walk to the two hides picking up en route Goldcrest and Redwing. From the hides, we saw Roe Deer, Teal, Oystercatcher, Snipe, Grey Heron and a small flock of Swallow . Late afternoon, after a tiring day of travel, we headed to our accommodation for the holiday, Ballintean Mountain Lodge, fabulously located in beautiful Glenfeshie. -

Studies of Less Familiar Birds 116. Crested Lark by I
Studies of less familiar birds 116. Crested Lark By I. J. Ferguson-Lees Photographs by lb Trap-Lind (Plates 6-7) WE HAVE ONLY two photographs of the Crested Lark (Galerida cristatd) here, but they show the salient features very well. The species gets its name from the long upstanding crest which arises from the middle of its crown and which is conspicuous even when depressed. Many other larks have crests and inexperienced observers are sometimes misled by that on the Skylark (Alauda arvensis) when they see it raised and at close range, but the crest of the Crested Lark is really of almost comical proportions. The two plates also illustrate several other characters of this species and attention is drawn to these in the caption on plate 7. Crested Larks have a somewhat undulating flight, rather like that of Woodlarks (Lislbfa arborea), and a characteris tic outline from their short tails and broad, rounded wings. In Britain the Crested Lark is a surprisingly rare vagrant which has been recorded on less than fifteen occasions. The last two accepted observations were on Fair Isle in 1952 (Brit. Birds, 46: 211) and in Devonin 1958-5C) (Brit. Birds, 53: 167, 422), though it should be added that the species has almost certainly also occurred in Kent on at least two occasions in the last five years. Thus, while so many other birds formerly regarded as very rare wanderers to Britain are now known to be of annual occurrence in small numbers—the Melodious Warbler (Hippolais polyglottd) is one such example—the enormous increase in experienced observers has failed to raise the number of records of the Crested Lark.