Testing Darwin's Hypothesis About The
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

1 "Principles of Phylogenetics: Ecology
"PRINCIPLES OF PHYLOGENETICS: ECOLOGY AND EVOLUTION" Integrative Biology 200 Spring 2016 University of California, Berkeley D.D. Ackerly March 7, 2016. Phylogenetics and Adaptation What is to be explained? • What is the evolutionary history of trait x that we see in a lineage (homology) or multiple lineages (homoplasy) - adaptations as states • Is natural selection the primary evolutionary process leading to the ‘fit’ of organisms to their environment? • Why are some traits more prevalent (occur in more species): number of origins vs. trait- dependent diversification rates (speciation – extinction) Some high points in the history of the adaptation debate: 1950s • Modern Synthesis of Genetics (Dobzhansky), Paleontology (Simpson) and Systematics (Mayr, Grant) 1960s • Rise of evolutionary ecology – synthesis of ecology with strong adaptationism via optimality theory, with little to no history; leads to Sociobiology in the 70s • Appearance of cladistics (Hennig) 1972 • Eldredge and Gould – punctuated equilibrium – argue that Modern Synthesis can’t explain pervasive observation of stasis in fossil record; Gould focuses on development and constraint as explanations, Eldredge more on ecology and importance of migration to minimize selective pressure 1979 • Gould and Lewontin – Spandrels – general critique of adaptationist program and call for rigorous hypothesis testing of alternatives for the ‘fit’ between organism and environment 1980’s • Debate on whether macroevolution can be explained by microevolutionary processes • Comparative methods -

Princeton USG Senate Meeting 14 May 9, 2020 9:30PM (EST)
Princeton USG Senate Meeting 14 May 9, 2020 9:30PM (EST) Introduction 1. Question and Answer Session (15 minutes) 2. President’s Report (5 minutes) New Business 1. Wintercession Presentation: Judy Jarvis (25 minutes) 2. Honor Committee Member Confirmations: Christian Potter (5 minutes) 3. Committee on Discipline Member Confirmations: Christian Potter (5 minutes) 4. RRR Referendum Position Paper: Andres Larrieu and Allen Liu (15 minutes) 5. CPUC Meeting Recap: Sarah Lee and Allen Liu (10 minutes) Honor Committee Re-Appointment Bios: Michael Wang ’21: The Honor Committee is excited to reappoint Michael Wang ’21, a junior from Carmel, Indiana who is concentrating in Math. On campus, he is involved with Army ROTC and is a Wilson PAA. Michael wants to continue serving on the Committee because he believes that the next few years of Committee work as we set new precedent and work through the challenges of reform are very important. He also believes it is important that the committee have more experienced members who can help guide the newer members. Samuel Fendler ‘21: The Honor Committee is excited to reappoint Samuel Fendler ’21. Samuel is a junior from New Jersey who is concentrating in Politics. On campus, he has been involved with the Mock Trial Team and the Princeton Student Veteran Group, where he currently holds the position of President. After graduating high school in 2011, he enlisted in the U.S. Marine Corps, where he served on Active Duty for five years as an Infantry Rifleman in Afghanistan, Romania, Norway, Finland, and Serbia, among other countries. He spent his final year as a Warfighting Instructor at The Basic School in Quantico, VA. -

MEN's Glee CLUB Giving the Function
WILLAMETTE-C. P. S. --- STADIUM, SATURDAY, NOV. 3, 10 O'CLOCK THE TRAIL OFF ICIAL RUBLICATION OF THE ASSOCIATED STUDENTS OF THE COLLEGE OF PUGET SOUND VOLUME-------------------------------- II ----------------------- TACOMA, WASHINGTON, W.EDNESDAY, OCTOBER 31,--------------------- 1923 ------------------------------NUMBER 7. KNIGHTS OF THE LOG MOST IlYIPORTANT GAMES LOGGERS liOLD HUSKIES TO SCORE INITIATE PLEDGES FOR ,C. P . S. COMING TO COACH MacNEAL Between ha lves at the fooball game The most importa nt game!'> of. the OF 24~0 IN SATURDAY'S BIG GANIE Saturday, the Knights of the Log With the wonderful showing made year a1·e coming this week and n ext by the football team lnst Satmday initiated their 14 freshman pledges. week. The game Saturdr.y will be and thE' praise given to the team, The ceremony w::ts perf ot·med on the in the Stadium a gains •. Will::u:wtte F ig hting ngainst one of the great- line to punt. The pass from center center of the fif.::l'd. The Knights were should go due tribute to Coach R. University. est football machines in the West, was 1ow, and be:fo1·e he could 1·ecover assisted by the Ladies of the S,plin- W. McNeal. With but enough men the lighter, less experienced Logger s the ball it had rolled across the goal ter. They have a !'. !~; t team weighing to make our team, McNeal turned of the College of Puget Sound held line where Hall of the Huskies fell When the teams le.ft the field af- about the san,c as our log·gcrs. -

A New Carnivorous Plant Lineage (Triantha) with a Unique Sticky-Inflorescence Trap
A new carnivorous plant lineage (Triantha) with a unique sticky-inflorescence trap Qianshi Lina,b,1, Cécile Anéc,d, Thomas J. Givnishc, and Sean W. Grahama,b aDepartment of Botany, University of British Columbia, Vancouver, BC V6T 1Z4, Canada; bUBC Botanical Garden, University of British Columbia, Vancouver, BC V6T 1Z4, Canada; cDepartment of Botany, University of Wisconsin–Madison, Madison, WI 53706; and dDepartment of Statistics, University of Wisconsin–Madison, Madison WI 53706 Edited by Elizabeth A. Kellogg, Donald Danforth Plant Science Center, St. Louis, MO, and approved June 5, 2021 (received for review October 30, 2020) Carnivorous plants consume animals for mineral nutrients that and in wetlands, including bogs, marly shorelines, and calcareous enhance growth and reproduction in nutrient-poor environments. spring-fed fens. In bogs, T. occidentalis is commonly found with Here, we report that Triantha occidentalis (Tofieldiaceae) represents recognized carnivorous plants such as Drosera rotundifolia a previously overlooked carnivorous lineage that captures insects on (Droseraceae) and Pinguicula vulgaris (Lentibulariaceae). During sticky inflorescences. Field experiments, isotopic data, and mixing the summer flowering season, T. occidentalis produces leafless models demonstrate significant N transfer from prey to Triantha, erect flowering stems up to 80 cm tall (12). These scapes have with an estimated 64% of leaf N obtained from prey capture in sticky glandular hairs, especially on their upper portions, a feature previous years, comparable to levels inferred for the cooccurring distinguishing Triantha from other genera of Tofieldiaceae round-leaved sundew, a recognized carnivore. N obtained via carnivory (Fig. 1). Small insects are often found trapped by these hairs; is exported from the inflorescence and developing fruits and may herbarium specimens are frequently covered in insects (Fig. -

Effects of Poaching, Habitat Destruction, and Climate Change on Nepenthes
Effects of Poaching, Habitat Destruction, and Climate Change On Nepenthes Lucas Barron 4/27/19 Effects of Poaching, Habitat Destruction, and Climate Change On Nepenthes Lucas Barron; 4/27/2018 1 Effects of Poaching, Habitat Destruction, and Climate Change On Nepenthes When Dominick Gravine, and avid Nepenthes collector and seller, visited Borneo in March of 2013 to trek up the slopes of mount Trusmadi, he not only saw the endangered Nepenthes species that inhabit the misty slopes, but also the effects of the rapid urbanization of the local villages. He saw ‘palm oil plantations as far as the eye Dominick Gravine and his collection of Nepenthes can see.’ (-Gravine). “Palm oil plantations completely clear the land of its natural biodiversity.’ (-Gravine). “While in the local villages, I was offered many plants which were obviously taken from the wild. The locals see these plants as a source of money. The over collect and sell them to collectors.” (-Gravine) While climbing the mountains, he saw many seed stalks cut of many plants from people along the trails, which has a large impact on these plants’ ability to reproduce. Habitat destruction and excessive poaching is having a severe Nepenthes Veitchii 'Candy Dreams'; created by Dominick Gravine effect on plant species not only in places like Borneo, but also worldwide. Although many people deny human caused endangerment of plant species due to excessive poaching, reckless destruction of habitats globally, and climate change, Effects of Poaching, Habitat Destruction, and Climate Change On Nepenthes Lucas Barron; 4/27/2018 2 has a profound, and rather severe, effect on plant species globally, but especially in extremely delicate regions of the world, such as the Indonesian islands. -

Carnivorous Plant Newsletter of Australia
Volume 6 September, 1980. Registered as a Publication, Category B. Page 6 CPNA Page 1 EDITORS, : : : : KEN HATLEY. SUSAN HATLEY. CORRESPONDENCE ADDRESSED TO : C.P.N.A. Wandena Rd, B~allsbrook East. West Australia. 6084. Published Quarterly by C.P.N.A. Subscriptions $6-OO Annually. Back copies issued to late subscribers. ........................................................................... FROM THE EDITORS. We would like to start this issue with an apology for its late arrival to you. Unfortunately, we experienced an unexpected delay in the arrival of replacment parts for the copying equipment. The good news this issue is of course that spring is upon us again, and with it the regrowth of our dormant varieties ready to come forward and show off their beauty for yet another year. The response for articles for the newsletter is dissappointing to say the least. There is enough experienced c.p. growers in Australia capable of contributing write-ups to ensure the success of the CPNA for many years to come, but if the lack of interest continues the newsletter is doomed to failure. To put it bluntly, if you want your newsletter to continue then get behind it and give it your support. Good Growing, Editors. ........................................................................... ........................................................................... FRONT COVER - DROSERA GLANDULIGERA, HABITAT WEST AUSTRALIA. Vol 6 CPNA Page 2 C.P. LETTERBOX. Received a letter from Mrs I.D. Anderson of 23 Harrow St, Launceston, Tasmania 7250 asking of other members in Tasmania. As we don't now publish a list of subscribers we leave it to any member in her area to drop a line and swap C.P. -
Certificate of Analysis
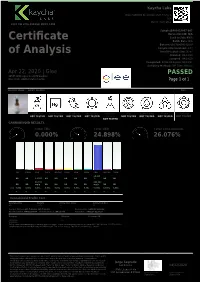
Kaycha Labs Glee 7,000 MG Broad Spectrum Tincture N/A Matrix: Derivative 4131 SW 47th AVENUE SUITE 1408 Sample:DA00416007-007 Harvest/Lot ID: N/A Certificate Seed to Sale #N/A Batch Date :N/A Batch#: GBS700004142020 Sample Size Received: 4 ml of Analysis Retail Product Size: 30 ml Ordered : 04/14/20 Sampled : 04/14/20 Completed: 04/22/20 Expires: 04/22/21 Sampling Method: SOP Client Method Apr 22, 2020 | Glee PASSED 10555 W Donges Court Milwaukee Wisconsin, United States 53224 Page 1 of 1 PRODUCT IMAGE SAFETY RESULTS MISC. Pesticides Heavy Metals Microbials Mycotoxins Residuals Filth Water Activity Moisture Terpenes NOT TESTED NOT TESTED NOT TESTED NOT TESTED Solvents NOT TESTED NOT TESTED NOT TESTED NOT TESTED NOT TESTED CANNABINOID RESULTS Total THC Total CBD Total Cannabinoids 0.000% 24.898% 26.076% CBC CBGA CBG THCV D8-THC CBDV CBN CBDA CBD D9-THC THCA 24.898 ND ND 1.178% ND ND ND ND ND % ND ND 11.780 248.980 ND ND mg/g ND ND ND ND ND mg/g ND ND LOD 0.001 0.001 0.001 0.001 0.001 0.001 0.001 0.001 0.0001 0.0001 0.001 % % % % % % % % % % % Cannabinoid Profile Test Analyzed by Weight Extraction date : Extracted By : 450 0.1065g NA NA Analysis Method -SOP.T.40.020, SOP.T.30.050 Reviewed On - 04/22/20 15:36:44 Analytical Batch -DA011837POT Instrument Used : DA-LC-003 Batch Date : 04/21/20 11:36:57 Reagent Dilution Consums. ID 042120.R21 400 042120.R20 Full spectrum cannabinoid analysis utilizing High Performance Liquid Chromatography with UV detection (HPLC-UV). -

Carnivorous Plant Newsletter Vol 48, No 2, June 2019
Byblis in cultivation in the tropics and in temperate climates Gregory Allan • Birmingham • United Kingdom • [email protected] Cindy Chiang • Singapore • [email protected] This article has been written based mostly on the authors’ experiences in growing Byblis in the UK and in Singapore. It is hoped that growers in regions with different climates will be able to extrapo- late from the information provided here, adjusting the methods described below to suit their own growing conditions. Hopefully much of the information provided here is universal in its application. Introduction If any genus of carnivorous plants deserves to be better known, both by horticulturalists and botanists, it is Byblis. The common name (ironically rarely used by enthusiasts) for the genus is “rainbow plants”, on account of the prismatic effect that can be produced when the sun shines on their copiously-produced sticky glands. All species follow a basic morphological plan: they have a central stem from which radiate numerous filiform leaves and scapes with showy flowers that typi- cally have purple petals (although white forms of most species are known) and vivid yellow anthers. Virtually all parts of the plants are covered in mucilage-tipped stalked glands (colloquially referred to as “tentacles”), which efficiently capture small insects, as well as microscopic sessile digestive glands. Another interesting characteristic of the genus is the presence of pulvini in most, if not all, species. Pulvini are swellings at the base of pedicels which, usually after successful pollination, uti- lize hydraulics to bend the pedicel, and consequently the developing fruit, downwards towards the ground. This phenomenon was discovered by Brian Barnes in the early 21st century. -

US, JAPANESE, and UK TELEVISUAL HIGH SCHOOLS, SPATIALITY, and the CONSTRUCTION of TEEN IDENTITY By
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by British Columbia's network of post-secondary digital repositories BLOCKING THE SCHOOL PLAY: US, JAPANESE, AND UK TELEVISUAL HIGH SCHOOLS, SPATIALITY, AND THE CONSTRUCTION OF TEEN IDENTITY by Jennifer Bomford B.A., University of Northern British Columbia, 1999 THESIS SUBMITTED IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF MASTER OF ARTS IN ENGLISH UNIVERSITY OF NORTHERN BRITISH COLUMBIA August 2016 © Jennifer Bomford, 2016 ABSTRACT School spaces differ regionally and internationally, and this difference can be seen in television programmes featuring high schools. As television must always create its spaces and places on the screen, what, then, is the significance of the varying emphases as well as the commonalities constructed in televisual high school settings in UK, US, and Japanese television shows? This master’s thesis considers how fictional televisual high schools both contest and construct national identity. In order to do this, it posits the existence of the televisual school story, a descendant of the literary school story. It then compares the formal and narrative ways in which Glee (2009-2015), Hex (2004-2005), and Ouran koukou hosutobu (2006) deploy space and place to create identity on the screen. In particular, it examines how heteronormativity and gender roles affect the abilities of characters to move through spaces, across boundaries, and gain secure places of their own. ii TABLE OF CONTENTS Abstract ii Table of Contents iii Acknowledgement v Introduction Orientation 1 Space and Place in Schools 5 Schools on TV 11 Schools on TV from Japan, 12 the U.S., and the U.K. -

Spring 2014 for Web.Pub
Spring 2014 Page 1 Botanic Garden News The Botanic Garden Volume 17, No. 1 of Smith College Spring 2014 Orchidelirium at Smith Madelaine Zadik K aren Yu ’16 is a STRIDE scholar who has worked with me since 2012 on educational projects and exhibitions at the Botanic Garden. As a first year, Karen learned about the workings of the Botanic Garden, our plant collections, and our various educational activities, including exhibit production. The plan was that by the end of her two-year STRIDE placement, she would produce an exhibit of her own. She had to hit the ground running, as the exhibit Botanical Printing: Artful Collaborations on Paper and Cloth opened just a month and a half after her arrival. Karen wrote label copy and press releases, learned mounting techniques, helped design the gallery layout with our modular walls, and learned how to use our unique hanging system in the Church Exhibition Gallery. For our next exhibit, From Petals to Paper: Poetic Inspiration from Flowers, Karen was involved in the many months of planning leading up to the installation in March 2013. When given a choice of possible exhibit topics for her own project, Karen chose to use a collection of orchid prints by artist Florence Helen Woolward to create an exhibit that would help illuminate the world of orchids for the visiting public. The prints are from Thesaurus Woolwardiae, Orchids of the Marquis of Lothian, which contained reproductions of 60 of Woolward’s orchid paintings. Woolward produced an impressive body of work, especially considering that she was never formally trained in either art or botany. -

Exapting Exaptation
Spotlight Exapting exaptation 1 2 3 3 Greger Larson , Philip A. Stephens , Jamshid J. Tehrani , and Robert H. Layton 1 Durham Evolution and Ancient DNA, Department of Archaeology, Durham University, South Road, Durham, DH1 3LE, UK 2 School of Biological and Biomedical Sciences, Durham University, South Road, Durham, DH1 3LE, UK 3 Department of Anthropology, Durham University, South Road, Durham, DH1 3LE, UK The term exaptation was introduced to encourage biol- As a result, all adaptations can also be said to be exapta- ogists to consider alternatives to adaptation to explain tions, thus rendering the term redundant [4]. the origins of traits. Here, we discuss why exaptation has proved more successful in technological than biological Technology and the exaptation of exaptation contexts, and propose a revised definition of exaptation Despite failing to catch on in evolutionary biology, exapta- applicable to both genetic and cultural evolution. tion has been adopted with considerable success in studies of the history of technology [5]. Technological innovations frequently involve the use of a process or artefact in a new The rise and fall of biological exaptation context [6]. A classic example is microwave radiation, Last year marked a decade since the death of Stephen Jay which was originally used in the radar magnetron to Gould, and 30 years since the publication of one of his most intercept and reflect off target objects, and was subse- provocative challenges to orthodox evolutionary theory quently exapted as a means to heat food. Similarly, tech- [1,2]. Concerned about a perceived lack of rigour, Gould, nologies that were initially developed as part of NASA’s together with Elizabeth Vrba, introduced a vocabulary space research program were later exploited for new com- intended to undermine the primacy of adaptation for mercial uses. -

Drama Audition Male/Female Junior Monologues
Drama Audition Male/Female Junior Monologues Northmead Creative & Performing Arts High School Classical and contemporary audition pieces. Imagine, Endeavour , A c h i e v e Northmead CAPAHS Campbell Street Northmead N S W 2 1 5 2 02 96304116 P r i n c i p a l - N . V a z q u e z Northmead Creative & Performing Arts High- Drama Audition The following pieces have been chosen from standard editions of the works. You may use the equivalent monologue from a different edition of the play, for example, if you have access to a different edition of the Shakespeare plays. For translated works, we have chosen a particular translation. However, you may use another translation if that is the version available to you. If you cannot access the Australian plays through your local library, bookshop or bookshops on our suggested list, published editions of the Australian plays are generally available through Currency Press. Audition Process You will be required to choose one monologue from the list provided to perform. Please note the delivery time of a monologue may vary depending on your interpretation of the chosen piece. Usual estimated time is between three to eight minutes. So please make sure your monologue is within this time frame. You may be asked to deliver your chosen piece more than once. You will also be tested for improvisation skills. So be prepared to use your imagination and creativity. A script may be handed to you during the audition. So be prepared for a cold read and once again use your imagination in showing how you would interpret the script reading.