Bulk Drug Substances Nominated for Use in Compounding Under Section 503B of the Federal Food, Drug, and Cosmetic Act
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The National Drugs List
^ ^ ^ ^ ^[ ^ The National Drugs List Of Syrian Arab Republic Sexth Edition 2006 ! " # "$ % &'() " # * +$, -. / & 0 /+12 3 4" 5 "$ . "$ 67"5,) 0 " /! !2 4? @ % 88 9 3: " # "$ ;+<=2 – G# H H2 I) – 6( – 65 : A B C "5 : , D )* . J!* HK"3 H"$ T ) 4 B K<) +$ LMA N O 3 4P<B &Q / RS ) H< C4VH /430 / 1988 V W* < C A GQ ") 4V / 1000 / C4VH /820 / 2001 V XX K<# C ,V /500 / 1992 V "!X V /946 / 2004 V Z < C V /914 / 2003 V ) < ] +$, [2 / ,) @# @ S%Q2 J"= [ &<\ @ +$ LMA 1 O \ . S X '( ^ & M_ `AB @ &' 3 4" + @ V= 4 )\ " : N " # "$ 6 ) G" 3Q + a C G /<"B d3: C K7 e , fM 4 Q b"$ " < $\ c"7: 5) G . HHH3Q J # Hg ' V"h 6< G* H5 !" # $%" & $' ,* ( )* + 2 ا اوا ادو +% 5 j 2 i1 6 B J' 6<X " 6"[ i2 "$ "< * i3 10 6 i4 11 6! ^ i5 13 6<X "!# * i6 15 7 G!, 6 - k 24"$d dl ?K V *4V h 63[46 ' i8 19 Adl 20 "( 2 i9 20 G Q) 6 i10 20 a 6 m[, 6 i11 21 ?K V $n i12 21 "% * i13 23 b+ 6 i14 23 oe C * i15 24 !, 2 6\ i16 25 C V pq * i17 26 ( S 6) 1, ++ &"r i19 3 +% 27 G 6 ""% i19 28 ^ Ks 2 i20 31 % Ks 2 i21 32 s * i22 35 " " * i23 37 "$ * i24 38 6" i25 39 V t h Gu* v!* 2 i26 39 ( 2 i27 40 B w< Ks 2 i28 40 d C &"r i29 42 "' 6 i30 42 " * i31 42 ":< * i32 5 ./ 0" -33 4 : ANAESTHETICS $ 1 2 -1 :GENERAL ANAESTHETICS AND OXYGEN 4 $1 2 2- ATRACURIUM BESYLATE DROPERIDOL ETHER FENTANYL HALOTHANE ISOFLURANE KETAMINE HCL NITROUS OXIDE OXYGEN PROPOFOL REMIFENTANIL SEVOFLURANE SUFENTANIL THIOPENTAL :LOCAL ANAESTHETICS !67$1 2 -5 AMYLEINE HCL=AMYLOCAINE ARTICAINE BENZOCAINE BUPIVACAINE CINCHOCAINE LIDOCAINE MEPIVACAINE OXETHAZAINE PRAMOXINE PRILOCAINE PREOPERATIVE MEDICATION & SEDATION FOR 9*: ;< " 2 -8 : : SHORT -TERM PROCEDURES ATROPINE DIAZEPAM INJ. -

[Product Monograph Template
PRODUCT MONOGRAPH INCLUDING PATIENT MEDICATION INFORMATION Kinevac® (Sincalide) For Injection 5 mcg/vial Diagnostic Cholecystokinetic Bracco Imaging Canada Date of Initial Approval: Montreal, Quebec July 2, 1996 Canada, H1J 2Z4 Date of Revision: October 30, 2019 Submission Control No: 231066 Product Monograph Kinevac® Page 1 of 14 TABLE OF CONTENTS TABLE OF CONTENTS ............................................................................................................. 2 PART I: HEALTH PROFESSIONAL INFORMATION ................................................................. 3 1 INDICATIONS ................................................................................................................. 3 Pediatrics ........................................................................................................................ 3 Geriatrics ......................................................................................................................... 3 2 CONTRAINDICATIONS .................................................................................................. 3 3 DOSAGE AND ADMINISTRATION ................................................................................ 4 Recommended Dose and Dosage Adjustment ................................................................ 4 Reconstitution .................................................................................................................. 4 4 OVERDOSAGE .............................................................................................................. -

Title 16. Crimes and Offenses Chapter 13. Controlled Substances Article 1
TITLE 16. CRIMES AND OFFENSES CHAPTER 13. CONTROLLED SUBSTANCES ARTICLE 1. GENERAL PROVISIONS § 16-13-1. Drug related objects (a) As used in this Code section, the term: (1) "Controlled substance" shall have the same meaning as defined in Article 2 of this chapter, relating to controlled substances. For the purposes of this Code section, the term "controlled substance" shall include marijuana as defined by paragraph (16) of Code Section 16-13-21. (2) "Dangerous drug" shall have the same meaning as defined in Article 3 of this chapter, relating to dangerous drugs. (3) "Drug related object" means any machine, instrument, tool, equipment, contrivance, or device which an average person would reasonably conclude is intended to be used for one or more of the following purposes: (A) To introduce into the human body any dangerous drug or controlled substance under circumstances in violation of the laws of this state; (B) To enhance the effect on the human body of any dangerous drug or controlled substance under circumstances in violation of the laws of this state; (C) To conceal any quantity of any dangerous drug or controlled substance under circumstances in violation of the laws of this state; or (D) To test the strength, effectiveness, or purity of any dangerous drug or controlled substance under circumstances in violation of the laws of this state. (4) "Knowingly" means having general knowledge that a machine, instrument, tool, item of equipment, contrivance, or device is a drug related object or having reasonable grounds to believe that any such object is or may, to an average person, appear to be a drug related object. -

Management of Cutaneous Hemangiomas in Pediatric Patients
PEDIATRIC DERMATOLOGY Series Editor: Camila K. Janniger, MD Management of Cutaneous Hemangiomas in Pediatric Patients Maria Letizia Musumeci, MD, PhD; Karina Schlecht, MD, PhD; Rosario Perrotta, MD; Robert A. Schwartz, MD, MPH; Giuseppe Micali, MD Cutaneous hemangiomas (CHs) are common benign during the first year of life and slow involution that vascular tumors of childhood. Clinically, they are usually is completed by 5 to 10 years of age.1 For characterized by a typical evolution profile, consist- this reason, no treatment is necessary in most cases. ing of a rapid proliferation during the first year of However, when CHs are located in areas at risk for life and slow involution that usually is completed functional complications; are of considerable size; or by 5 to 10 years of age. In most cases, no treat- repeatedly undergo bleeding, ulceration, or superin- ment is necessary. However, when CHs are located fection, a prompt and adequate treatment approach in areas at risk for functional complications; are of is required.2 considerable size; or repeatedly undergo bleeding, ulceration, or superinfection, a prompt and adequate Epidemiology treatment approach is required. First-line approaches CHs are present in 1.0% to 2.6% of neonates and in include topical, intralesional, and systemic corti- 10% to 12% of infants by 12 months of age.3 Thirty costeroids. Second-line options include interferon percent of CHs are first evident at birth; the remain- alfa-2a and -2b, laser therapy, and surgical therapy. der appear during the second month of life. The Third-line approaches include cytotoxins, emboliza- frequency of these benign tumors increases in pre- tion, and angiogenesis inhibitors. -

Pruritus: Scratching the Surface
Pruritus: Scratching the surface Iris Ale, MD Director Allergy Unit, University Hospital Professor of Dermatology Republic University, Uruguay Member of the ICDRG ITCH • defined as an “unpleasant sensation of the skin leading to the desire to scratch” -- Samuel Hafenreffer (1660) • The definition offered by the German physician Samuel Hafenreffer in 1660 has yet to be improved upon. • However, it turns out that itch is, indeed, inseparable from the desire to scratch. Savin JA. How should we define itching? J Am Acad Dermatol. 1998;39(2 Pt 1):268-9. Pruritus • “Scratching is one of nature’s sweetest gratifications, and the one nearest to hand….” -- Michel de Montaigne (1553) “…..But repentance follows too annoyingly close at its heels.” The Essays of Montaigne Itch has been ranked, by scientific and artistic observers alike, among the most distressing physical sensations one can experience: In Dante’s Inferno, falsifiers were punished by “the burning rage / of fierce itching that nothing could relieve” Pruritus and body defence • Pruritus fulfils an essential part of the innate defence mechanism of the body. • Next to pain, itch may serve as an alarm system to remove possibly damaging or harming substances from the skin. • Itch, and the accompanying scratch reflex, evolved in order to protect us from such dangers as malaria, yellow fever, and dengue, transmitted by mosquitoes, typhus-bearing lice, plague-bearing fleas • However, chronic itch lost this function. Chronic Pruritus • Chronic pruritus is a common and distressing symptom, that is associated with a variety of skin conditions and systemic diseases • It usually has a dramatic impact on the quality of life of the affected individuals Chronic Pruritus • Despite being the major symptom associated with skin disease, our understanding of the pathogenesis of most types of itch is limited, and current therapies are often inadequate. -
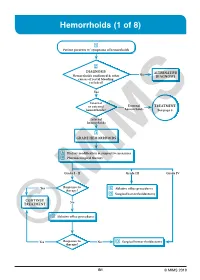
Hemorrhoids (1 of 8)
Hemorrhoids (1 of 8) 1 Patient presents w/ symptoms of hemorrhoids 2 DIAGNOSIS No ALTERNATIVE Hemorrhoids confi rmed & other DIAGNOSIS causes of rectal bleeding excluded? Yes Internal or external External TREATMENT hemorrhoids? hemorrhoids See page 3 Internal hemorrhoids 3 GRADE HEMORRHOIDS A Dietary modifi cation & supportive measures B Pharmacological therapy Grade I - II Grade III Grade IV Yes Response to C Ablative offi ce procedures therapy? D Surgical hemorrhoidectomy CONTINUE No TREATMENT C Ablative offi ce procedures Yes Response to No D Surgical hemorrhoidectomy ©therapy? MIMS B1 © MIMS 2019 Hemorrhoids (2 of 8) 1 SYMPTOMS ATTRIBUTED TO HEMORRHOIDS • Rectal bleeding - Most common presenting symptom - Bright red blood which may drip or squirt into the toilet bowl or scanty amounts may be seen on toilet tissue • Discomfort due to rectal protrusion or lump • Anal pain • HEMORRHOIDS Anal itching 2 DIAGNOSIS Medical History • Assess nature, duration & severity of symptoms - Ask about bleeding, its amount & frequency - Ask about presence of prolapsing tissue, its timing & reproducibility • Elicit possible risk factors for development of hemorrhoidal symptoms - Low-fi ber diets cause small-caliber stools, resulting in straining during defecation & engorgement of hemorrhoids - Prolonged sitting on a toilet which may cause a problem in the venous return in the perianal area - Pregnancy - Advanced age • Th e signs & symptoms of hemorrhoids are not specifi c to the disease, so care must be taken to avoid missing other causes of pathology • Obtain -

Chapter 12 Monographs of 99Mtc Pharmaceuticals 12
Chapter 12 Monographs of 99mTc Pharmaceuticals 12 12.1 99mTc-Pertechnetate I. Zolle and P.O. Bremer Chemical name Chemical structure Sodium pertechnetate Sodium pertechnetate 99mTc injection (fission) (Ph. Eur.) Technetium Tc 99m pertechnetate injection (USP) 99m ± Pertechnetate anion ( TcO4) 99mTc(VII)-Na-pertechnetate Physical characteristics Commercial products Ec=140.5 keV (IT) 99Mo/99mTc generator: T1/2 =6.02 h GE Healthcare Bristol-Myers Squibb Mallinckrodt/Tyco Preparation Sodium pertechnetate 99mTc is eluted from an approved 99Mo/99mTc generator with ster- ile, isotonic saline. Generator systems differ; therefore, elution should be performed ac- cording to the manual provided by the manufacturer. Aseptic conditions have to be maintained throughout the operation, keeping the elution needle sterile. The total eluted activity and volume are recorded at the time of elution. The resulting 99mTc ac- tivity concentration depends on the elution volume. Sodium pertechnetate 99mTc is a clear, colorless solution for intravenous injection. The pH value is 4.0±8.0 (Ph. Eur.). Description of Eluate 99mTc eluate is described in the European Pharmacopeia in two specific monographs de- pending on the method of preparation of the parent radionuclide 99Mo, which is generally isolated from fission products (Monograph 124) (Council of Europe 2005a), or produced by neutron activation of metallic 98Mo-oxide (Monograph 283) (Council of Europe 2005b). Sodium pertechnetate 99mTc injection solution satisfies the general requirements of parenteral preparations stated in the European Pharmacopeia (Council of Europe 2004). The specific activity of 99mTc-pertechnetate is not stated in the Pharmacopeia; however, it is recommended that the eluate is obtained from a generator that is eluted regularly, 174 12.1 99mTc-Pertechnetate every 24 h. -

(12) United States Patent (10) Patent No.: US 6,803,046 B2 Metcalfe Et Al
USOO6803046B2 (12) United States Patent (10) Patent No.: US 6,803,046 B2 Metcalfe et al. (45) Date of Patent: Oct. 12, 2004 (54) SINCALIDE FORMULATIONS OTHER PUBLICATIONS (75) Inventors: Edmund C. Metcalfe, Hillsborough, NJ Sitzmann, et al., “Cholecystokinin Prevents Parenteral (US); Jo Anna Monteferrante, Raritan Nutrition Induced Biliary Sludge in Humans.” Surgery, Township, NJ (US); Margaret Gynecology & Obstetrics, vol. 170, Jan. 1990, pp. 25-31. Newborn, Hamilton Township, NJ Teitelbaum et al., “Treatment of Parenteral Nutrition-ASSo (US); Irene Ropiak, Lawrenceville, NJ ciated Cholestasis with Cholecystokinin-Octapeptide,” (US); Ernst Schramm, North Journal of Pediatric Surgery, vol. 30, No. 7, Jul. 1995, pp. Brunswick, NJ (US); Gregory W. 1082-1085. White, Monmouth Junction, NJ (US); Moss and Amii, “New Approaches to Understanding the Julius P. Zodda, Mercerville, NJ (US) Etiology and Treatment of Total Parenteral Nutrition-ASSo ciated Cholestasis,” Seminars in Pediatric Surgery, vol. 8, (73) Assignee: Bracco International B.V., Amsterdam No. 3, Aug. 1999, pp. 140–147. (NL) Teitelbaum, "Parenteral Nutrition-ASSociated Cholestasis,” c: - Current Opinion in Pediatrics, vol. 9, 1997, pp. 270–275. (*) Notice: Subject to any State the SME, tly Teitelbaum and Tracy, “Parenteral Nutrition-Associated patent is extended or adjusted under Cholestasis,” Seminars in Pediatric Surgery, vol. 10, No. 2, U.S.C. 154(b) by 0 days. May 2001, pp. 72–80. Strickley, “Parenteral Formulations of Small Molecules (21) Appl. No.: 10/222,540 Therapeutics Marketed in the United States (1999) -Part (22) Filed: Aug. 16, 2002 1, PDA Journal of Pharmaceutical Science & Technology, e - Vs vol. 53, No. 6, Nov.-Dec. 1999, pp. -
![Ehealth DSI [Ehdsi V2.2.2-OR] Ehealth DSI – Master Value Set](https://docslib.b-cdn.net/cover/8870/ehealth-dsi-ehdsi-v2-2-2-or-ehealth-dsi-master-value-set-1028870.webp)
Ehealth DSI [Ehdsi V2.2.2-OR] Ehealth DSI – Master Value Set
MTC eHealth DSI [eHDSI v2.2.2-OR] eHealth DSI – Master Value Set Catalogue Responsible : eHDSI Solution Provider PublishDate : Wed Nov 08 16:16:10 CET 2017 © eHealth DSI eHDSI Solution Provider v2.2.2-OR Wed Nov 08 16:16:10 CET 2017 Page 1 of 490 MTC Table of Contents epSOSActiveIngredient 4 epSOSAdministrativeGender 148 epSOSAdverseEventType 149 epSOSAllergenNoDrugs 150 epSOSBloodGroup 155 epSOSBloodPressure 156 epSOSCodeNoMedication 157 epSOSCodeProb 158 epSOSConfidentiality 159 epSOSCountry 160 epSOSDisplayLabel 167 epSOSDocumentCode 170 epSOSDoseForm 171 epSOSHealthcareProfessionalRoles 184 epSOSIllnessesandDisorders 186 epSOSLanguage 448 epSOSMedicalDevices 458 epSOSNullFavor 461 epSOSPackage 462 © eHealth DSI eHDSI Solution Provider v2.2.2-OR Wed Nov 08 16:16:10 CET 2017 Page 2 of 490 MTC epSOSPersonalRelationship 464 epSOSPregnancyInformation 466 epSOSProcedures 467 epSOSReactionAllergy 470 epSOSResolutionOutcome 472 epSOSRoleClass 473 epSOSRouteofAdministration 474 epSOSSections 477 epSOSSeverity 478 epSOSSocialHistory 479 epSOSStatusCode 480 epSOSSubstitutionCode 481 epSOSTelecomAddress 482 epSOSTimingEvent 483 epSOSUnits 484 epSOSUnknownInformation 487 epSOSVaccine 488 © eHealth DSI eHDSI Solution Provider v2.2.2-OR Wed Nov 08 16:16:10 CET 2017 Page 3 of 490 MTC epSOSActiveIngredient epSOSActiveIngredient Value Set ID 1.3.6.1.4.1.12559.11.10.1.3.1.42.24 TRANSLATIONS Code System ID Code System Version Concept Code Description (FSN) 2.16.840.1.113883.6.73 2017-01 A ALIMENTARY TRACT AND METABOLISM 2.16.840.1.113883.6.73 2017-01 -

Drugs and Biologicals Fee Schedule
Payment Allowance Limits for PEIA Effective January 1, 2013 through December 31, 2013 The absence or presence of a HCPCS code does not indicate PEIA coverage. Similarily, the inclusion of a payment limit does not indicate coverage by PEIA. processing the claim. Vaccine Infusion DME Infusion Blood Clotting HCPCS Code Short Description HCPCS Code Dosage Payment Limit AWP% Vaccine Limit AWP% Limit AWP% Blood limit Factor Notes 90371 Hep b ig, im 1 ML$ 104.73 90375 Rabies ig, im/sc 150 IU$ 206.47 90376 Rabies ig, heat treated 150 IU$ 197.46 90385 Rh ig, minidose, im 50 MCG$ 24.62 90585 Bcg vaccine, percut 50 MG$ 120.87 90586 Bcg vaccine, intravesical 1 EACH$ 120.87 90632 Hep a vaccine, adult im 1 ML$ 50.93 90654 Flu vaccine, intradermal, no preserv 0.1 ML$ 18.98 95 18.981 90655 Flu vaccine no preserv 6-35m, im 0.25 ML$ 16.46 95 16.456 90656 Flu vaccine no preserv 3 yo & >, im 0.50 ML$ 12.40 95 12.398 90657 Flu vaccine, 6-35 mo, im 0.25 ML$ 6.02 95 6.023 90660 Flu vaccine, nasal 0.2 ML$ 23.46 95 23.456 90662 Flu vacc prsv free inc antig 0.5 ML$ 30.92 95 30.923 90669 Pneumococcal vacc, 7 val im 0.5 ML$ 95.48 95 95.481 90670 Pneumococcal vacc, 13 val im 0.5 ML$ 137.03 95 137.028 90675 Rabies vaccine, im 1 ML$ 190.40 90691 Typhoid vaccine, im 0.5 ML$ 68.27 90703 Tetanus vaccine, im 0.5 ML$ 35.41 90714 Td vaccine no prsrv >/= 7 yo, im 0.5 ML$ 19.93 90715 Tdap => 7 yo, im 0.5 ML$ 33.35 90717 Yellow fever vaccine, sc 0.5 ML$ 71.49 90732 Pneumococcal vaccine 0.5 ML$ 65.77 95 65.774 90733 Meningococcal vaccine, sc 0.5 ML$ 106.49 90735 Encephalitis -

Diagnosis and Treatment of Varicose Veins in the Legs
Diagnosis and treatment of varicose veins in the legs KCE reports 164C Belgian Health Care Knowledge Centre Federaal Kenniscentrum voor de Gezondheidszorg Centre fédéral d’expertise des soins de santé 2011 The Belgian Health Care Knowledge Centre Introduction: The Belgian Health Care Knowledge Centre (KCE) is an organization of public interest, created on the 24th of December 2002 under the supervision of the Minister of Public Health and Social Affairs. KCE is in charge of conducting studies that support the political decision making on health care and health insurance. Executive Board Actual Members: Pierre Gillet (President), Dirk Cuypers (Vice-president), Jo De Cock (Vice-president), Frank Van Massenhove (Vice-president), Maggie De Block, Jean-Pierre Baeyens, Ri de Ridder, Olivier De Stexhe, Johan Pauwels, Daniel Devos, Jean-Noël Godin, Xavier De Cuyper, Paul Palstermans, Xavier Brenez, Rita Thys, Marc Moens, Marco Schetgen, Patrick Verertbruggen, Michel Foulon, Myriam Hubinon, Michael Callens, Bernard Lange, Jean-Claude Praet. Substitute Members: Rita Cuypers, Christiaan De Coster, Benoît Collin, Lambert Stamatakis, Karel Vermeyen, Katrien Kesteloot, Bart Ooghe, Frederic Lernoux, Leo Neels, Greet Musch, Geert Messiaen, Anne Remacle, Roland Lemeye, Annick Poncé, Pierre Smiets, Jan Bertels, Celien Van Moerkerke, Yolande Husden, Ludo Meyers, Olivier Thonon, François Perl. Government commissioner: Yves Roger Management Chief Executive Officer: Raf Mertens Assistant Chief Executive Officer: Jean-Pierre Closon Information Federaal Kenniscentrum -

Basic Skin Care and Topical Therapies for Atopic Dermatitis
REVIEWS Basic Skin Care and Topical Therapies for Atopic Dermatitis: Essential Approaches and Beyond Sala-Cunill A1*, Lazaro M2*, Herráez L3, Quiñones MD4, Moro-Moro M5, Sanchez I6, On behalf of the Skin Allergy Committee of Spanish Society of Allergy and Clinical Immunology (SEAIC) 1Allergy Section, Internal Medicine Department, Hospital Universitario Vall d'Hebron, Barcelona, Spain 2Allergy Department, Hospital Universitario de Salamanca, Alergoasma, Salamanca, Spain 3Allergy Department, Hospital Universitario 12 de Octubre, Madrid, Spain 4Allergy Section, Hospital Monte Naranco, Oviedo, Spain 5Allergy Department, Hospital Universitario Fundación Alcorcón, Alcorcón, Madrid, Spain 6Clínica Dermatología y Alergia, Badajoz, Spain *Both authors contributed equally to the manuscript J Investig Allergol Clin Immunol 2018; Vol. 28(6): 379-391 doi: 10.18176/jiaci.0293 Abstract Atopic dermatitis (AD) is a recurrent and chronic skin disease characterized by dysfunction of the epithelial barrier, skin inflammation, and immune dysregulation, with changes in the skin microbiota and colonization by Staphylococcus aureus being common. For this reason, the therapeutic approach to AD is complex and should be directed at restoring skin barrier function, reducing dehydration, maintaining acidic pH, and avoiding superinfection and exposure to possible allergens. There is no curative treatment for AD. However, a series of measures are recommended to alleviate the disease and enable patients to improve their quality of life. These include adequate skin hydration and restoration of the skin barrier with the use of emollients, antibacterial measures, specific approaches to reduce pruritus and scratching, wet wrap applications, avoidance of typical AD triggers, and topical anti-inflammatory drugs. Anti-inflammatory treatment is generally recommended during acute flares or, more recently, for preventive management.