Antisedan ® (Atipamezole Hydrochloride)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Anesthesia and Analgesia in Laboratory Animals
GUIDELINES ON ANESTHESIA AND ANALGESIA IN LABORATORY ANIMALS University of South Florida provides the following guidelines for use by IACUC-certified faculty and staff. CONTENTS PAGE A. Background……………………………………………………….…………………………… 1 B. Definitions....……………………………………………………..…………………………….. 2 C. General Considerations……………………………………….,…………………………….. 3 D. Controlled Substances……………………………………….……………………………… 3 E. Pre-Anesthetic Treatments………………………………….………………………………. 4 F. General Anesthetics………………………………………….………………………………. 4 G. Neuromuscular Blocking Agents………………………….……………………………….. 5 H. Monitoring Anesthesia…………………………………….…………………………………. 6 I. Analgesics……………………………………………………………………………………… 7 J. Comments regarding Anesthetics and Analgesics……………………………………... 7 REFERENCE TABLES PAGE I. Signs of Pain and Distress in Laboratory Animals………………………………………… 10 II. Commonly Used Anesthetics and Analgesics for Mice….………..…...….………...…… 11 III. Commonly Used Anesthetics and Analgesics for Rats……………………………...…… 12 IV. Commonly Used Anesthetics and Analgesics for Gerbils……….……………..…….. 13 V. Commonly Used Anesthetics and Analgesics for Hamsters…….……………..……. 14 VI. Commonly Used Anesthetics and Analgesics for Guinea Pigs….…………….….……. 15 VII. Commonly Used Anesthetics and Analgesics for Rabbits.……...…………….………… 16 VIII. Commonly Used Anesthetics and Analgesics for Dogs.…………………….…………… 17 IX. Commonly Used Anesthetics and Analgesics for Cats.……………………..…………… 18 X. Commonly Used Anesthetics and Analgesics for Pigs ..……………..….………………..19 XI. Commonly Used Anesthetics and Analgesics -

Psychedelics in Psychiatry: Neuroplastic, Immunomodulatory, and Neurotransmitter Mechanismss
Supplemental Material can be found at: /content/suppl/2020/12/18/73.1.202.DC1.html 1521-0081/73/1/202–277$35.00 https://doi.org/10.1124/pharmrev.120.000056 PHARMACOLOGICAL REVIEWS Pharmacol Rev 73:202–277, January 2021 Copyright © 2020 by The Author(s) This is an open access article distributed under the CC BY-NC Attribution 4.0 International license. ASSOCIATE EDITOR: MICHAEL NADER Psychedelics in Psychiatry: Neuroplastic, Immunomodulatory, and Neurotransmitter Mechanismss Antonio Inserra, Danilo De Gregorio, and Gabriella Gobbi Neurobiological Psychiatry Unit, Department of Psychiatry, McGill University, Montreal, Quebec, Canada Abstract ...................................................................................205 Significance Statement. ..................................................................205 I. Introduction . ..............................................................................205 A. Review Outline ........................................................................205 B. Psychiatric Disorders and the Need for Novel Pharmacotherapies .......................206 C. Psychedelic Compounds as Novel Therapeutics in Psychiatry: Overview and Comparison with Current Available Treatments . .....................................206 D. Classical or Serotonergic Psychedelics versus Nonclassical Psychedelics: Definition ......208 Downloaded from E. Dissociative Anesthetics................................................................209 F. Empathogens-Entactogens . ............................................................209 -

Veterinary Anesthetic and Analgesic Formulary 3Rd Edition, Version G
Veterinary Anesthetic and Analgesic Formulary 3rd Edition, Version G I. Introduction and Use of the UC‐Denver Veterinary Formulary II. Anesthetic and Analgesic Considerations III. Species Specific Veterinary Formulary 1. Mouse 2. Rat 3. Neonatal Rodent 4. Guinea Pig 5. Chinchilla 6. Gerbil 7. Rabbit 8. Dog 9. Pig 10. Sheep 11. Non‐Pharmaceutical Grade Anesthetics IV. References I. Introduction and Use of the UC‐Denver Formulary Basic Definitions: Anesthesia: central nervous system depression that provides amnesia, unconsciousness and immobility in response to a painful stimulation. Drugs that produce anesthesia may or may not provide analgesia (1, 2). Analgesia: The absence of pain in response to stimulation that would normally be painful. An analgesic drug can provide analgesia by acting at the level of the central nervous system or at the site of inflammation to diminish or block pain signals (1, 2). Sedation: A state of mental calmness, decreased response to environmental stimuli, and muscle relaxation. This state is characterized by suppression of spontaneous movement with maintenance of spinal reflexes (1). Animal anesthesia and analgesia are crucial components of an animal use protocol. This document is provided to aid in the design of an anesthetic and analgesic plan to prevent animal pain whenever possible. However, this document should not be perceived to replace consultation with the university’s veterinary staff. As required by law, the veterinary staff should be consulted to assist in the planning of procedures where anesthetics and analgesics will be used to avoid or minimize discomfort, distress and pain in animals (3, 4). Prior to administration, all use of anesthetics and analgesic are to be approved by the Institutional Animal Care and Use Committee (IACUC). -

Dr Michael Crowley Professor Malcolm Dando
DOWN THE SLIPPERY SLOPE? A STUDY OF CONTEMPORARY DUAL-USE CHEMICAL AND LIFE SCIENCE RESEARCH POTENTIALLY APPLICABLE TO INCAPACITATING CHEMICAL AGENT WEAPONS BIOCHEMICAL SECURITY 2030 POLICY PAPER SERIES NUMBER 8 BIOCHEMICAL SECURITY 2030 PROJECT BRADFORD NON-LETHAL WEAPONS RESEARCH PROJECT OCTOBER 2014 Dr Michael Crowley Project Coordinator of the Bradford Non-Lethal Weapons Research Project based at the Peace Studies Department, School of Social and International Studies, University of Bradford, United Kingdom. Email: [email protected] Professor Malcolm Dando School of Social and International Studies University of Bradford, Bradford Email: [email protected] ACKNOWLEDGEMENTS The authors, as well as the Biochemical Security 2030 Project organisers, would like to thank those who have reviewed or commented upon drafts of this report. In particular this includes Professor Julian Perry Robinson and Dr Ralf Trapp as well as others, including those members of the Biochemical Security 2030 expert panel, who commented on this document. We are also grateful to those Government officials, named and unnamed, who have replied to our information requests and/or commented upon specific sections of this report. We are grateful to the Economic and Social Research Council as well as the Defence Science and Technology Laboratory Futures and Innovation Domain for funding the Biochemical Security 2030 Project. The authors would also like to express their gratitude to the Joseph Rowntree Charitable Trust for their financial support for aspects of this research. The research findings and policy recommendations detailed in this publication have been developed under the auspices of the Bradford Non-Lethal Weapons Research Project (BNLWRP) and reflect the organisation's position on these issues. -

Pain Management in Patients with Substance-Use Disorders
Pain Management in Patients with Substance-Use Disorders By Valerie Prince, Pharm.D., FAPhA, BCPS Reviewed by Beth A. Sproule, Pharm.D.; Jeffrey T. Sherer, Pharm.D., MPH, BCPS; and Patricia H. Powell, Pharm.D., BCPS Learning Objectives regarding drug interactions with illicit substances or prescribed pain medications. Finally, there are issues 1. Construct a therapeutic plan to overcome barri- ers to effective pain management in a patient with related to the comorbidities of the patient with addic- addiction. tion (e.g., psychiatric disorders or physical concerns 2. Distinguish high-risk patients from low-risk patients related to the addiction) that should influence product regarding use of opioids to manage pain. selection. 3. Design a treatment plan for the management of acute pain in a patient with addiction. Epidemiology 4. Design a pharmacotherapy plan for a patient with Pain is the second most common cause of work- coexisting addiction and chronic noncancer pain. place absenteeism. The prevalence of chronic pain may 5. Design a pain management plan that encompasses be much higher among patients with substance use dis- recommended nonpharmacologic components for orders than among the general population. In the 2006 a patient with a history of substance abuse. National Survey on Drug Use and Health, past-year alco- hol addiction or abuse occurred in 10.3% of men and 5.1% of women. In the same survey, 12.3% of men and Introduction 6.3% of women were reported as having a substance-use Pain, which is one of the most common reasons disorder (abuse or addiction) during the past year. -

The Pharmacokinetics of Medetomidine Administered Subcutaneously During Isoflurane Anaesthesia in Sprague-Dawley Rats
animals Article The Pharmacokinetics of Medetomidine Administered Subcutaneously during Isoflurane Anaesthesia in Sprague-Dawley Rats Leila T. Kint 1, Bhedita J. Seewoo 2,3,4 , Timothy H. Hyndman 5 , Michael W. Clarke 6, Scott H. Edwards 7, Jennifer Rodger 3,4, Kirk W. Feindel 2,8 and Gabrielle C. Musk 1,9,* 1 Faculty of Health and Medical Sciences, The University of Western Australia, Perth 6009, Australia; [email protected] 2 Centre for Microscopy, Characterisation and Analysis, Research Infrastructure Centres, The University of Western Australia, Perth 6009, Australia; [email protected] (B.J.S.); [email protected] (K.W.F.) 3 Experimental and Regenerative Neurosciences, School of Biological Sciences, The University of Western Australia, Perth 6009, Australia; [email protected] 4 Brain Plasticity Group, Perron Institute for Neurological and Translational Science, Perth 6009, Australia 5 School of Veterinary Medicine, Murdoch University, Perth 6150, Australia; [email protected] 6 Metabolomics Australia, Centre for Microscopy, Characterisation and Analysis, The University of Western Australia, Perth 6009, Australia; [email protected] 7 School of Animal Veterinary Sciences, Charles Sturt University, Wagga Wagga 2650, Australia; [email protected] 8 School of Biomedical Sciences, the University of Western Australia, Perth 6009, Australia 9 Animal Care Services, the University of Western Australia, Perth 6009, Australia * Correspondence: [email protected] Received: 28 May 2020; Accepted: 17 June 2020; Published: 18 June 2020 Simple Summary: Rodents, including rats, are used as animal models for research investigating neurological diseases in humans. To enable this research the animals are anaesthetized to facilitate imaging of the brain, but the anaesthetic drugs impact the results of the research. -

Effect of Intramuscular Medetomidine Administration on Tear Flow in Rats
veterinary sciences Communication Effect of Intramuscular Medetomidine Administration on Tear Flow in Rats Teppei Kanda 1,2,* , Yuka Mizoguchi 2, Kayo Furumoto 1,2, Yuki Shimizu 1,2, Noritaka Maeta 1,2 and Toshinori Furukawa 3 1 Faculty of Veterinary Medicine, Okayama University of Science, 1-3 Ikoino-oka, Imabari, Ehime 794-8555, Japan 2 Department of Comparative Animal Science, College of Life Science, Kurashiki University of Science and the Arts, 2640 Nishinoura, Tsurajima-cho, Kurashiki, Okayama 712-8505, Japan 3 Department of Animal Pharmaceutical Science, School of Pharmaceutical Science, Kyushu University of Health and Welfare, 1714-1 Yoshino-cho, Nobeoka, Miyazaki 882-8508, Japan * Correspondence: [email protected] Received: 19 March 2020; Accepted: 10 April 2020; Published: 13 April 2020 Abstract: Medetomidine has been reported to decrease tear flow significantly in dogs, cats, and pigs when used as a sedative or analgesic; however, there are no such reports when it comes to rats. The present study aimed to investigate the effect of medetomidine on tear flow in rats. Medetomidine in doses of 50, 100, or 200 µg/kg or a physiological saline solution as the control, were administered intramuscularly to male Slc:Wistar/ST rats. After the administration of medetomidine, tear flow in both eyes was measured using a phenol red thread tear test. The area under the curve (AUC) of phenol red thread test values from baseline to 8 h was calculated. Data were plotted against the dose of medetomidine and simple linear regression analysis was performed. The effect of the drug on phenol red thread test values was considered dose-related when linear analysis yielded a significant relationship. -

SEPARATION ANXIETY – HOME ALONE Kenneth M. Martin, DVM, DACVB Veterinary Behavior Consultations, LLC,& TEAM Education in A
SEPARATION ANXIETY – HOME ALONE Kenneth M. Martin, DVM, DACVB Veterinary Behavior Consultations, LLC,& TEAM Education in Animal Behavior, LLC, Spicewood, Tx Separation anxiety is perhaps one of the most common behavioral problems of dogs and it has been documented in cats.1 Approximately 20% of dogs in the United States suffer from some form of separation anxiety or distress.2 Its presentation is damaging to the human-animal bond and may result in relinquishment and/or euthanasia. Problem duration may be negatively associated with outcome, therefore it is important to screen for behavioral signs and symptoms during routine veterinary wellness visits. Clients may not always be forthcoming with information regarding their pet’s behavior. Early identification and intervention may truly mean the difference between life and death. While addressing behavioral concerns in private practice can be time consuming, preserving the bond and keeping patients in the home can be a significant income generator. Most cases of separation anxiety can be addressed with regard to treatment recommendations within only 30 minutes of doctor-client-patient time. Ideally, medical history, preliminary behavioral history and blood testing is done prior to the behavior appointment to allow for honing in the diagnosis and specific treatment recommendations. Signalment, Predisposition, and Risk Factors Canine cases may be biased toward male dogs which represent 60-70 percent of the patients in most studies.3 Approximately 50 percent of patients are mixed breed and they may be considered at increased risk verses purebred dogs.3 Mixed breeds may reflect where the dogs are most often sourced. Dogs from shelters or rescue may be at increased risk and they may be more resistant to improvement with behavioral therapy.4 Often dogs with separation anxiety were at one point relinquished or had more than one owner. -

Summary of Product Characteristics
Health Products Regulatory Authority Summary of Product Characteristics 1 NAME OF THE VETERINARY MEDICINAL PRODUCT SEDATOR, 1.0 mg/ml, solution for injection for cats and dogs 2 QUALITATIVE AND QUANTITATIVE COMPOSITION 1 ml contains: Active substance Medetomidine hydrochloride 1.0 mg Excipients Methyl parahydroxybenzoate (E 218) 1.0 mg Propyl parahydroxybenzoate 0.2 mg For the full list of excipients, see section 6.1 3 PHARMACEUTICAL FORM Solution for injection. Clear and colourless solution. 4 CLINICAL PARTICULARS 4.1 Target Species Dogs, cats. 4.2 Indications for use, specifying the target species Dogs: for restraint, sedation and analgesia associated with clinical examinations and procedures, minor surgery, pre-anaesthesia and as a premedication before thiopentone-halothane general anaesthesia and as a premedicant before general anaesthesia with propofol. In combination with butorphanol for sedation, analgesia and as a premedicant to thiopentone anaesthesia. Cats: for restraint and sedation. In combination with ketamine for induction of general anaesthesia prior to surgical procedures in the cat. In combination with butorphanol for sedation and analgesia, and combined with both butorphanol and ketamine for general anaesthesia. As a premedication before alphaxalone/alphadolone for general anaesthesia. 4.3 Contraindications Do not use in conjunction with sympathomimetic amines. Care should be taken with the use of medetomidine in animals with cardiovascular disease or in poor general health. Before using any combinations consult the contraindications and warnings that appear on the other products’ data sheet. Medetomidine should not be used with thiopentone or propofol in animals with cardiac or respiratory disease (see also section 4.10). 4.4 Special warnings for each target species An appropriately graduated syringe must be used to allow accurate administration of the required dose volume. -

Neuropathic Pain
WSAVA Global Pain Council Pain Management Protocol The following pain management protocol is tiered to ensure a global relevance, recognizing that not all analgesic modalities are available to veterinary practitioners and vary from region to region around the world. Its implementation will be guided by the various analgesic modalities available along with the needs of the individual patient requiring treatment. This protocol is reproduced from the WSAVA Global Pain Treatise, a succinct yet comprehensive review of pain assessment, various pain modalities, and the treatment of various clinically painful scenarios in both dogs and cats. The WSAVA GPC Pain Treatise published in the Journal of Small Animal Practice and is available for open access at the GPC pages of www.wsava.org. Neuropathic pain Neuropathic pain requires several classes of medications and procedures as it cannot be adequately managed with a single pharmacological or non-pharmacological therapy. Prior to, and during any surgical procedure, various dierent analgesic drugs and modalities can be used to reduce the inciting nociceptive aerent impulse. Many of these are continued postoperatively to reduce both peripheral (PNS) and central (CNS) sensitization. NSAIDs ere is evidence to support an inammatory response driving the pathophysiological changes of the peripheral and central nervous systems resulting in neuropathic pain and augmentation of pain processing by spinal prostanoids. While no studies are reported at this time, human clinical trials are currently underway investigating various modalities to target specic components of the neuroinammatory process. It is advised that NSAIDs be used in the treatment of neuropathic pain. Opioids Opioids may be included in a multimodal regimen to manage neuropathic pain, but not as a stand alone analgesic. -
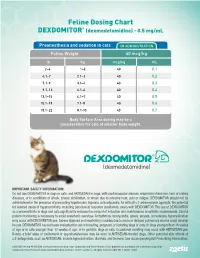
Feline Dosing Chart DEXDOMITOR® (Dexmedetomidine)
Feline Dosing Chart ® DEXDOMITOR (dexmedetomidine) - 0.5 mg/mL Preanesthesia and sedation in cats IM ADMINISTRATION Feline Weight 40 mcg/kg lb kg mcg/kg mL 2–4 1–2 40 0.1 4.1–7 2.1–3 40 0.2 7.1–9 3.1–4 40 0.3 9.1–13 4.1–6 40 0.4 13.1–15 6.1–7 40 0.5 15.1–18 7.1–8 40 0.6 18.1–22 8.1–10 40 0.7 Body Surface Area dosing may be a consideration for cats of smaller body weight. IMPORTANT SAFETY INFORMATION: Do not use DEXDOMITOR in dogs or cats, and ANTISEDAN in dogs, with cardiovascular disease, respiratory disorders, liver or kidney diseases, or in conditions of shock, severe debilitation, or stress due to extreme heat, cold or fatigue. DEXDOMITOR should not be administered in the presence of preexisting hypotension, hypoxia, or bradycardia. As with all α2-adrenoceptor agonists, the potential for isolated cases of hypersensitivity, including paradoxical response (excitation), exists with DEXDOMITOR. The use of DEXDOMITOR as a preanesthetic in dogs and cats significantly reduces the amount of induction and maintenance anesthetic requirements. Careful patient monitoring is necessary to avoid anesthetic overdose. Arrhythmias, bradycardia, apnea, emesis, convulsions, hypersalivation may occur with DEXDOMITOR use. Severe dyspnea and respiratory crackles due to acute or delayed pulmonary edema could develop in cats. DEXDOMITOR has not been evaluated for use in breeding, pregnant, or lactating dogs or cats; in dogs younger than 16 weeks of age or in cats younger than 12 weeks of age; or in geriatric dogs or cats. -

AVMA Guidelines for the Euthanasia of Animals: 2020 Edition*
AVMA Guidelines for the Euthanasia of Animals: 2020 Edition* Members of the Panel on Euthanasia Steven Leary, DVM, DACLAM (Chair); Fidelis Pharmaceuticals, High Ridge, Missouri Wendy Underwood, DVM (Vice Chair); Indianapolis, Indiana Raymond Anthony, PhD (Ethicist); University of Alaska Anchorage, Anchorage, Alaska Samuel Cartner, DVM, MPH, PhD, DACLAM (Lead, Laboratory Animals Working Group); University of Alabama at Birmingham, Birmingham, Alabama Temple Grandin, PhD (Lead, Physical Methods Working Group); Colorado State University, Fort Collins, Colorado Cheryl Greenacre, DVM, DABVP (Lead, Avian Working Group); University of Tennessee, Knoxville, Tennessee Sharon Gwaltney-Brant, DVM, PhD, DABVT, DABT (Lead, Noninhaled Agents Working Group); Veterinary Information Network, Mahomet, Illinois Mary Ann McCrackin, DVM, PhD, DACVS, DACLAM (Lead, Companion Animals Working Group); University of Georgia, Athens, Georgia Robert Meyer, DVM, DACVAA (Lead, Inhaled Agents Working Group); Mississippi State University, Mississippi State, Mississippi David Miller, DVM, PhD, DACZM, DACAW (Lead, Reptiles, Zoo and Wildlife Working Group); Loveland, Colorado Jan Shearer, DVM, MS, DACAW (Lead, Animals Farmed for Food and Fiber Working Group); Iowa State University, Ames, Iowa Tracy Turner, DVM, MS, DACVS, DACVSMR (Lead, Equine Working Group); Turner Equine Sports Medicine and Surgery, Stillwater, Minnesota Roy Yanong, VMD (Lead, Aquatics Working Group); University of Florida, Ruskin, Florida AVMA Staff Consultants Cia L. Johnson, DVM, MS, MSc; Director,