Minutes of the New Haven Meeting, June 23-25, 1932
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Henry Andrews Bumstead 1870-1920
NATIONAL ACADEMY OF SCIENCES OF THE UNITED STATES OF AMERICA BIOGRAPHICAL MEMOIRS VOLUME XIII SECOND MEMOIR BIOGRAPHICAL MEMOIR OF HENRY ANDREWS BUMSTEAD 1870-1920 BY LEIGH PAGE PRESENTED TO THE ACADEMY AT THE ANNUAL MEETING, 1929 HENRY ANDREWS BUMSTEAD BY LIUGH PAGE Henry Andrews Bumstead was born in the small town of Pekin, Illinois, on March 12th, 1870, son of Samuel Josiah Bumstead and Sarah Ellen Seiwell. His father, who was a physician of considerable local prominence, had graduated from the medical school in Philadelphia and was one of the first American students of medicine to go to Vienna to complete his studies. While the family was in Vienna, Bumstead, then a child three years of age, learned to speak German as fluently as he spoke English, an accomplishment which was to prove valuable to him in his subsequent career. Bumstead was descended from an old New England family which traces its origin to Thomas Bumstead, a native of Eng- land, who settled in Boston, Massachusetts, about 1640. Many of his ancestors were engaged in the professions, his paternal grandfather, the Reverend Samuel Andrews Bumstead, being a graduate of Princeton Theological Seminary and a minister in active service. From them he inherited a keen mind and an unusually retentive memory. It is related that long before he had learned to read, his Sunday school teacher surprised his mother by complimenting her on the ease with which her son had rendered the Sunday lesson. It turned out that his mother made a habit of reading the lesson to Bumstead before he left for school, and the child's remarkable performance there was due to his ability to hold in his memory every word of the lesson after hearing it read to him a single time. -

The Emission Theory of Electromagnetism
TRANSACTIONS OF THE CONNECTICUT ACADEMY OF ARTS AND SCIENCES VOLUME 26J JANUARY 1924 [PAGES 213-243 The Emission Theory of Electromagnetism BY LEIGH PAGE Prolessor 01 Matbematical Physics in Yale University NI!\V HAVEN, CONNECTICUT PUBLISHED BY THE CONNECTICUT ACADEMY OF ARTS AND SCIENCBS AND TO BE OBTAINBD ALSO PROM THE YALE UNIVERSITY PRESS THE EMISSION THEORY OF ELECTROMAGNETISM LEIGH PAGE HISTORICAL INTRODUCTION The subject of electroDlagnetism exhibits a remarkable dualisnt which is absent from other branches of physics. Not only do electric and magnetic fields appear as essentially distinct though related entities, but in the study of the effects produced by one electric charge on another it is necessary to distinguish between positive and negative electricity and in the case of magtletislD between north and south-seeking polarity. Naturally the first systematic investigations in the sltbject were limited to tile phenomena of magnetostatics and electrostatics, ill which the forces between statiollary magtlets or electric cllarges are tIle objects of study. As early as 1600 Gilbert explained tIle directive tendency of a freely suspended magnetic l1eedle as due to tIle magnetization of the earth, combined with the general princillie that like poles repel al1d unlike attract. However, it was 110t until two centuries later that Coulolnb, by means of the torsion balance, demonstrated that tile law of force, both as t-egards 111agnetic poles and electric charges, was the sanle il1verse square law tllat Newton 11ad fOUlld to hold in the province of gravitation. At the time of Coulomb's deattl no connection was knOW!l between the pllenonlena of magnetism and electricity. -

A Bibliography of Publications By, and About, Max Born
A Bibliography of Publications by, and about, Max Born Nelson H. F. Beebe University of Utah Department of Mathematics, 110 LCB 155 S 1400 E RM 233 Salt Lake City, UT 84112-0090 USA Tel: +1 801 581 5254 FAX: +1 801 581 4148 E-mail: [email protected], [email protected], [email protected] (Internet) WWW URL: http://www.math.utah.edu/~beebe/ 07 June 2021 Version 1.125 Title word cross-reference 1=r [DL08]. 137 [Bor35b]. $2.50 [Mor54]. $3.80 [Kle70c]. $4.95 [Kle70b]. 2 [Bor18g, Pac10]. n log n [ADO11]. 1 [Lor10]. 14.99/$25.00 [Ber04]. 1801 [vS21]. 1911 [Meh75]. 1916-1955 [Mar73]. 1926 [Bor54a]. 1928 [HKP+32a, HKP+32b]. 1933 [Kr¨o98]. 1945 [Bor45b]. 1947 [Bor63-28]. 1951 [Bor63b]. 1955 [For70]. 1957 [BBF+57a]. 1970 [KS71, Lin98]. 1995 [Kr¨o98]. 20 [Goe88]. 24.80 [Kle70a]. 240pp [Ber04]. 3.85 [Bro72]. 37th [Bor53c]. 4 [RSS+07]. 480pp [Ber04]. 4th [Bor50b]. 1 2 50th [Bor59b, Bor63v]. 55 [BB69a]. 60th [Ber15, Sho20]. 7 [Sho20]. 7th [Sho20]. 80th [Ano63, CS79b, Ros79]. 978 [Sho20]. 978-1-108-47743-7 [Sho20]. ˆın [Bor69f]. Abbildungsfehler [Bor32c]. aberrations [Bor32c]. Abhandlungen [BB22a, BB63g, Bor63c, Bor63d, Seg64]. Ableitung [Bor10b]. Ablenkung [vS21]. Abraham [BvL23, BvL63]. Absolute [BS35, BL18b, Bor18d, BL63b]. absoluten [Bor18d]. absorbance [BL11]. absorbierenden [BL11]. absorbing [BL11]. absorption [Bor32d]. Absorptionsbanden [Bor32d]. Absorptionsverm¨ogen [BL11]. Achievement [Bor68a]. Achtzehn [BBF+57b, ABH+55, ABH+87]. achtzigsten [Hei62]. Activity [Bor35c, Bor15d, Bor15e, Bor22e, Bor36a, Bor63-51, Bor63-52]. Adiabatenprinzip [Bor26b, Bor63j]. Adiabatensatzes [BF28, BF63b]. adiabatic [Bor26b, BF63b, BF28, Bor63j]. Adsorption [BW63a, BF30, BW31a, BW31b, BF63c]. -

A Complete Bibliography of Publications By, and About, James Clerk Maxwell
A Complete Bibliography of Publications by, and about, James Clerk Maxwell Nelson H. F. Beebe University of Utah Department of Mathematics, 110 LCB 155 S 1400 E RM 233 Salt Lake City, UT 84112-0090 USA Tel: +1 801 581 5254 FAX: +1 801 581 4148 E-mail: [email protected], [email protected], [email protected] (Internet) WWW URL: http://www.math.utah.edu/~beebe/ 10 July 2021 Version 1.35 Title word cross-reference 1=2 [Arm04]. $17.00 [Eve84]. $195 [Buc92]. $195.00 [Hun05a]. $25.00 [Mar99a]. $25.95 [Jam15]. $28.75 [Can90]. $285.00 [Hun05a]. $29.00 [Row05]. 3 [Ive05]. $315.00 [Hun05a]. $38.45 [Har93]. $39.50 [Sto82]. $45.00 [Ber08]. $49.50 [Hun92, Mar93]. $55 [She04a, She04b]. $59.95 [Yos99]. $6.95 [Eve84]. $77.00 [Sto82]. $77.00/$ [Tur83]. $8.95 [Lin71]. $86.25 [Can90]. B [Ive05]. E [Ive05]. [Spr06]. H [Dia94b]. [Spr06]. v [Cle70c]. &c. [Cle73b]. -theorem [Dia94b]. 0 [Cha84, Hun92, Mar99a, Pip99, Pip04, Row05, She04a, She04b]. 0-226-07882-5 [Roc87]. 0-226-07884-1 [Can90]. 0-226-07886-8 [Can90]. 1 2 0-226-87375-7 [Row05]. 0-470-86088-X [Hun06, Pip04]. 0-521-25625-9 [Buc92, Hun05a, Jam91, Seg92]. 0-521-25626-7 [Hun05a]. 0-521-25627-5 [Hun05a]. 0-521-35365-3 [Mar93, Hun92]. 0-521-56102-7 [Yos99, Pip99]. 0-679-43342-2 [Mar99a]. 0-7503-0759-5 [She04a, She04b]. 0-8014-2641-3 [Har93]. 0-85274-563-X [The87]. 0-86228-026-5 [Smi85, Cha84]. 0-86341-101-0 [Hun89]. 0-934223-34-3 [Har96]. -

Old/Past/Ancient/Historic Frontiers in Black Hole Astrophysics
UC Irvine UC Irvine Previously Published Works Title Old/Past/Ancient/Historic Frontiers in Black Hole Astrophysics Permalink https://escholarship.org/uc/item/7n17n98f Journal NEW FRONTIERS IN BLACK HOLE ASTROPHYSICS, 12(S324) ISSN 1743-9213 Author Trimble, Virginia Publication Date 2017 DOI 10.1017/S1743921317001983 License https://creativecommons.org/licenses/by/4.0/ 4.0 Peer reviewed eScholarship.org Powered by the California Digital Library University of California New Frontiers in Black Hole Astrophysics Proceedings IAU Symposium No. 324, 2016 c International Astronomical Union 2017 Andreja Gomboc, ed. doi:10.1017/S1743921317001983 Old/Past/Ancient/Historic Frontiers in Black Hole Astrophysics Virginia Trimble Department of Physics and Astronomy, University of California Irvine CA 92697 and Queen Jadwiga Observatory, Rzepiennik, Poland email: [email protected] Abstract. Basic questions about black holes, some of which are fairly old, include (1) What is a black hole? (2) Do black holes exist? And the answer to this depends a good deal on the answer to (1), (3) Where, when, why, and how have they formed? and (4) What are they good for? Here I attempt some elaboration of the questions and partial answers, noting that general relativity is required to described some of the phenomena, while dear old Isaac Newton is OK for others. Keywords. black hole, general relativity, horizon, gravitational redshift, binary stars, quasars, gamma-ray bursts, gravitational radiation, advection, singularity 1. Introduction The phrase “black hole” in its modern meaning first appeared in print more than 60 years ago (Ewing 1964). Bartusiak (2015) has chased the words back to their Princeton pairing and popularization by Robert Dicke and John Wheeler from 1963 onward. -

Yale Department of Physics Summer 2013
Yale Department of Physics Summer 2013 Greetings from the (Former) Department Chair — When I became Department Chair the Department of Energy, and with colleagues across the in 2007, I immediately began wider physics community, and during this time, the Tandem planning my first Newsletter. It accelerator was shut down. Ultimately (skipping over the is now 2013 and you are reading many details), this process culminated in the appointment of the one and only newsletter I’ve Karsten Heeger as a senior faculty member and new Director of managed to put together during my WNSL, as well as the junior appointment of Reina Maruyama term. It’s a sign that a lot of other (see article on faculty appointments on page 3). Together with things were happening, and as I colleagues in particle and AMO physics, WNSL now represents step down as Department Chair, I a center of excellence in weak interaction physics, as well as a am really pleased to tell you about leader in the development of detectors based on noble gases, all the wonderful things we have including the liquid Argon technology that will be used for accomplished, despite some challenging financial times. an eventual long-baseline neutrino oscillation experiment. My first priority as Chair was strategic planning for the The Physics Department grew and turned over significantly coming decade. Over a period of several months in the fall of over the past decade, bringing a lot of excitement and vitality. 2007, we held faculty discussions nearly every week. Six faculty My predecessor as Chair, Shankar, hired 16 new faculty, and I members gave overview talks, outlining the science landscape have hired 7. -
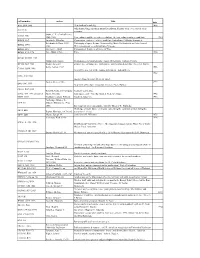
Call Number Author Title Date AG5 .B64 2006 New Book of Knowledge
call number author Title date AG5 .B64 2006 New book of knowledge. 2006 John Simon Guggenheim memorial foundation. Reports of the secretary & of the AS911.J6 treasurer. Snow, C. P. (Charles Percy), AZ361 .S56 1905-1980 Two cultures and the scientific revolution, the two cultures and a second look 1963 BD632 .F67 Formánek, Miloslav. Časoprostor v politice : některé problémy fyzikalismu / Miloslav Formánek. Reichenbach, Hans, 1891- Philosophy of space & time. Translated by Maria Reichenbach and John Freund. BD632 .R413 1953. With introductory remarks by Rudolf Carnap. BD632.G92 Gurnbaum, Adolf Philosophical Problems of Space of Time BD638 .P73 1996 Price, Huw, 1953- Price. 1996 BH301.N3 H55 1985 Hildebrandt, Stefan. Mathematics and optimal form / Stefan Hildebrandt, Anthony Tromba. BT130 .B69 1983 Brams, Steven J. omniscience, omnipotence, immortality, and incomprehensibility / Steven J. Brams. Berry, Adrian, 1937- 1996 CB161 .B459 1996 Next 500 years : life in the coming millennium / Adrian Berry. 1982 E158 .A48 1982 America from the road / Reader's digest. Forbes, Steve, 1947- 1999 E885 .F67 1999 New birth of freedom : vision for America / Steve Forbes. G1019 .R47 1955 Rand McNally and Company. Standard world atlas. G70.4 .S77 1992 oversized Strain, Priscilla. Looking at earth / Priscilla Strain & Frederick Engle. 1992 GB55 .G313 Gaddum, Leonard William, Harold L. Knowles. 1953 Fairbridge, Rhodes W. GC9 .F3 (Rhodes Whitmore), 1914- 2006. Encyclopedia of oceanography, edited by Rhodes W. Fairbridge. Challenge of man's future; an inquiry concerning the condition of man during the GF31 .B68 Brown, Harrison, 1917-1986. years that lie ahead. GF47 .M63 Moran, Joseph M. [and] James H. Wiersma. 1973 Anderson, Walt, 1933- 1996 GN281.4 .A53 1996 Evolution isn't what it used to be : the augmented animal and the whole wired world / Walter Truett Anderson. -

Yale Department of Physics Newsletter
Yale Department of Physics Newsletter Spring 2003 Greetings from the Chair — It is my pleasure to continue the tradition of the Physics Departmental Newsletter established by my predecessor, Professor Charles Baltay, in the summer of 2001, which also marked the beginning of my term. We are pleased to welcome several new faculty to the department. Professor Steven Girvin, a noted condensed matter theorist, joined us in July 2001. Steve was well known to all of us for his breadth and depth of interest in various areas of condensed matter physics, and when we became aware that he was considering relocation from Indiana University, we moved aggressively to bring him here, fighting off competition from some worthy opponents. Steve has already propelled our already strong condensed matter group to new heights. He also works closely with Applied Physics on problems related to quantum information, especially with Michel Devoret, another recent and marvelous addition to the Yale faculty in January 2002. In fact the only thing Steve does not know is the word “no,” and an obvious conflict of interest prevents me from rectifying the situation. Another prized addition to the department will be Professor Pierre Hohenberg, Channel surfing who will switch from Deputy Provost to Adjunct Professor at the end of this academic year. Pierre, who is a member of the National Academy of Sciences, has during physics class? made seminal contributions to condensed matter physics that have been recognized by a string of honors too long to mention here. So I will limit myself to the latest See page 5… two: the Planck Medal and the Onsager Prize. -

Yale Department of Physics Newsletter
Yale Department of Physics Newsletter Fall 2005 Greetings from the Chair — Assuming you were familiar with the department A Solar-Powered as described in the last Newsletter of Spring 2003, what would you notice if you dropped by What? for a visit? First, you will find me a lot happier than in this photograph. Of the many reasons let us begin with people. You would run into a lot of young and dynamic faculty. I will say just a few words about each since details follow later in the newsletter. Helen Caines, who was a Research Scientist at WNSL, joined the faculty as assistant professor effective July 2004. She is an emerging leader in the field of relativistic Heavy Ion Collisions. Eric Dufresne (mechanical engineering with a joint appointment in physics) works in soft condensed matter, often collaborating with Simon Mochrie. It is a pleasure to welcome Eric to the faculty after having had him as a (star) student in my class years ago. (He vehemently denies that he still owes me Problem Set 15, overdue by 10 years!) Richard Easther joined us in January 2004. He works in the interface between particle physics and cosmology and has Yale’s Team Lux runs with the already strengthened our ties to Astronomy. Bonnie Fleming joined us in fall 2004. Bonnie works on neutrinos and is a terrific pedagogue. Steven Furlanetto will start sun. See page 3. in January 2006 as assistant professor. Steve, who specializes in the re-ionization period of the early universe, is going to be central to our efforts in Astrophysics.